|
| Method no.: |
ID-110 |
| |
|
| Matrix: |
Air, Wipe (Smear Tabs for particulate
fluoride) |
| |
|
OSHA Permissible Exposure Limits
Final Rule Limits
Fluorides (as F):
Hydrogen
Fluoride: |
2.5 mg/m3 Time
Weighted Average (TWA)
3 ppm TWA
6 ppm Short-Term Exposure
Limit (STEL) |
| |
|
Transitional
Limits
Fluoride Dust (as
F):
Hydrogen Fluoride: |
2.5 mg/m3 TWA
3 ppm
TWA |
| |
|
Collection
Procedures
Fluorides:
Hydrogen Fluoride: |
A known volume of air is drawn
through a cassette containing a mixed-cellulose ester (MCE) filter
and backup pad using a calibrated personal sampling pump.
A
known volume of air is drawn through a three-piece
cassette containing a MCE filter and a sodium-carbonate
treated backup pad using a personal sampling pump. |
| |
|
Recommended Air
Volumes:
TWA Determinations
STEL Determinations |
90 L
22.5 L |
| |
|
| Recommended Sampling Rate: |
1.5 L/min |
| |
|
| Analytical Procedure: |
Filters (MCE) are fused with
sodium hydroxide, and treated back-up pads are desorbed
with deionized water. Analysis is performed using an ion specific
electrode and the method of standard additions. All samples are
analyzed for total fluoride content. |
| |
|
Detection Limit
Quantitative: |
25 µg (25-mL sample solution
volume) |
| |
|
Precision and Accuracy
Validation Range:
CV1
Bias
Overall Analytical Error |
350 to 700 µg load (as
HF)
0.057
-0.01
±12.4% |
| |
|
| Method Classification: |
Validated Analytical Method |
| |
|
| Date (Date Revised): |
December 1988 (Feb. 1991) |
| |
|
Commercial manufacturers and products mentioned in this method are
for descriptive use only and do not constitute endorsements by
USDOL-OSHA.
Similar products from other sources can
be substituted.
|
| |
|
Division of Physical Measurements and Inorganic
Analyses
OSHA Technical Center
Sandy, Utah
|
1.
Introduction
This method
describes the collection and analysis of airborne hydrogen fluoride
or particulate fluoride-containing compounds in the
workplace. It is applicable for both Short-Term (STEL)
and Time Weighted Average (TWA) exposure evaluations.
1.1. History
In the past, samples for
determination of particulate and gaseous fluoride compounds were
collected using the sampling procedure mentioned in this method
with one exception. For HF sampling, the MCE filter was previously
placed on the chemically-treated backup pad. The
filter and treated pad are now separated within a
three-piece cassette. Samples have always been
analyzed at the OSHA laboratory using an ion specific electrode
(ISE)/standard addition technique.
1.2. Principle
An air sample is taken by drawing a known amount of air
through a cassette containing a mixed-cellulose ester
(MCE) membrane filter for the collection of particulate fluoride
compounds. For the collection of hydrogen fluoride (HF) gas, a
sodium carbonate-treated back-up pad is
placed behind the MCE filter.
The MCE filter containing
particulate fluoride is fused with sodium hydroxide to facilitate
solubility of the particulate. The resulting alkali flux is dried,
neutralized with hydrochloric acid, and then diluted to a
specified volume with deionized water. The sodium
carbonate-treated back-up pad is
desorbed with deionized water. The pH of each sample solution is
adjusted to be within a pH of 4.5 to 10.5. Immediately before
analysis, each sample is combined with Tris-Tartrate
(T-T) complexing buffer solution. The concentration
of each sample is determined by a standard addition technique
using an ISE for fluoride.
1.3. Advantages and
Disadvantages
1.3.1. This method is a simple sampling and
analytical procedure able to detect fluoride over a large
concentration range.
1.3.2. Analytical interferences are
minimized by the addition of a buffer and use of the standard
additions technique. Ions commonly associated with fluoride in
work atmospheres (i.e. chloride, bromide, iodide, sulfate,
nitrate, phosphate, and acetate) do not interfere with the
analysis.
1.3.3. The instrumentation and sample
preparation are inexpensive.
1.3.4. A disadvantage of
this method is the sample preparation which involves a tedious
sample flux technique.
1.3.5. Another disadvantage is
the tendency for ISE readings to drift when measuring low
concentrations. 1.4. Physical and Chemical Properties
(8.1.)
Some properties and additional information
regarding hydrogen fluoride are listed below. Particulate fluoride
compounds are too numerous to describe; sodium fluoride (NaF) is
included below as an example of a soluble
fluoride-containing particulate.
| Synonyms |
(HF) |
Hydrofluoric acid, hydrogen
fluoride |
|
(Fluoride) |
Variety of compounds, fluoride ion,
perfluoride |
|
| CAS no. |
(HF) |
7664-39-3 |
|
(Fluoride) |
16984-48-8 |
|
(NaF) |
7681-49-4 |
|
| Physical properties |
(HF) |
Colorless, fuming mobile liquid. Attacks
glass and any silicon-containing
material. |
|
(NaF) |
Clear crystals or white powder |
|
| Boiling Point |
(HF 38% solution) |
112 °C |
|
(HF) |
19.5 °C |
|
(NaF) |
1695 °C |
|
| Density |
(HF) |
0.988 (liquid @ 14 °C) |
|
(NaF) |
2.558 (14 °C) |
|
| Solubility |
(HF) |
Soluble in water |
|
(NaF) |
Soluble in water |
|
| Physiologic effect |
(HF) |
Strong irritant to eyes, skin, and mucous
membranes. Toxic by inhalation or ingestion. |
|
|
(NaF) |
Tissue irritant, toxic by inhalation or
ingestion. |
|
| Uses |
(HF) |
Aluminum production, fluorocarbons,
stainless steel pickling, glass etching, oil well acidizing,
gasoline production, uranium processing. |
|
|
(NaF) |
Water fluoridation (~ 1 ppm), degassing
steel, fungicide and rodenticide, wood preservative,
electroplating, toothpaste additive, glass manufacture,
disinfectant (in fermentation), dental
prophylaxis. | 2. Range and Detection Limit
The analytical range is from 25 to 2,000 µg fluoride.
Samples larger than 2,000 µg can be diluted and analyzed. An
estimated detection limit of 25 µg is used. This detection limit is
based on the lowest concentration standard used in the analysis.
3. Method Performance
Quality control data (8.2.) from the OSHA Technical Center
(OSHA-SLTC) indicate an average recovery of 99% with a coefficient
of variation of 0.057 for 60 samples spiked with sodium fluoride.
The overall analytical error for these quality control samples
(analyzed from 1986 to 1990) was ±12.4%. Factors which may influence
precision and accuracy include electrode temperature, drift, and
noise.
The sampling portion of the method had previously
been evaluated at a flow rate of 1.5 L/min (see reference 8.3. for
more information).
4. Interferences
(Analytical) (8.4.)
Interference due to OH¯ ion can be
controlled by maintaining the pH between 4.5 and 10.5. Loss of
fluoride from complex formation of fluoride ion with polyvalent
cations is controlled by the addition of a buffer during analysis.
Interferences can further be minimized using a standard additions
technique. Formation of HF and HF¯ at low pH is avoided by using a
sample matrix having a pH > 4.5. Interferences from
complexing agents such as hydrogen ion (H+), aluminum,
silicon, or iron [Fe(3+)] are
minimized by using a buffer and a standard additions technique.
5. Sampling
5.1. Equipment - Air
Samples (Note: Any chemicals used in sampling media preparation
should be reagent grade or better)
5.1.1. Particulate collection: Mixed cellulose
ester (MCE) filters (0.8 µm pore size), cellulose backup pads
for filter support, and two- or three-piece
cassettes, 37-mm diameter, (part no. MAWP 037 A0,
Millipore Corp., Bedford, MA).
5.1.2.Particulate and hydrogen fluoride collection:
In addition to the MCE filter, a filter spacer (Cat. No.
225-23, SKC Inc., Eighty Four, PA) and a
chemically-treated backup pad is also used. The
spacer is a support ring for the MCE filter. The treated pad
ensures capture of HF. The backup pads are treated using the
following scheme:
- Forceps
- Pipets, 0.5 mL
- Sodium carbonate (Na2CO3)
- Glycerol (C3H8O3)
- Impregnation solution [Na2CO3
solution with glycerol]
- prepare by dissolving 4.0
g Na2CO3 in 50 mL deionized water, add 2
mL glycerol, and dilute this solution to 100 mL with deionized
water. Using a forceps, remove the
MCE filters from the three-piece cassettes and use the opened
cassettes as supports for backup pad impregnation. Each backup
pad should be resting on the ridge of the middle insert of the
cassette and not in contact with the cassette base when
impregnating.
Slowly pipet 0.5 mL of the impregnation
solution over the entire backup pad and let dry overnight.
Assemble the cassettes such that the backup pad resides in the
lower section (the cassette outlet), and the MCE filter and
filter spacer is in the upper section (the inlet) as
shown:
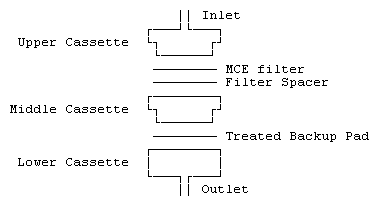
Use the treated backup pads within 2
months of preparation.
5.1.3. Gel bands (Omega Specialty
Instrument Co., Chelmsford, MA) for sealing cassettes. Seal
cassettes with the bands after assembly.
5.1.4. Sampling
pumps capable of sampling at 1.5 liters/min (L/min) with less
than ±5% pump error.
5.1.5. Assorted flexible tubing.
5.1.6. Stopwatch and bubble tube or meter for pump
calibration. 5.2.
Equipment - Wipe Samples
5.2.1. Smear tabs (part no.
225-24, SKC Inc., Eighty Four, PA).
5.2.2. Deionized
water.
5.2.3. Scintillation vials, 20-mL (part no. 74515
or 58515, Kimble, Div. of Owens-Illinois Inc.,
Toledo, OH) with polypropylene or Teflon cap liners.
5.3. Sampling Procedure
- Air Samples (Also see note in Section 7.3.)
5.3.1. Connect sampling media
for calibration purposes to each pump and calibrate to
approximately 1.5 L/min.
5.3.2. Remove the calibration
media and connect the appropriate sampling media to each pump.
For particulate fluoride sampling only, use the media described
in Section 5.1.1. For HF and particulate fluoride, use the media
listed in Section 5.1.2. Make sure the filter cassette is
connected to the pump with flexible tubing such that sampled air
enters the MCE filter first.
5.3.3. Place the sampling
assembly on the employee or workplace area so it does not
interfere with the work being performed.
5.3.4. Collect
air samples at a flow rate of 1.5 L/min. Whenever possible for
TWA measurements, take consecutive samples for 1 h each. Take
enough samples to cover the entire workshift. Observe each
cassette during sampling to make sure the filter does not become
overloaded.
5.3.5. Take samples for at least 15 minutes
for STEL measurements. The minimum suggested total air volume
for STEL determinations is 22.5 L.
5.3.6. Replace the
end plugs into the filter cassettes immediately after sampling.
5.3.7. Securely wrap each sample cassette end-to-end
with an OSHA Form 21 sample seal. 5.4. Sampling Procedure - Wipe
Samples
5.4.1. Wear clean, impervious,
disposable gloves when taking each wipe sample.
5.4.2.
Moisten the wipe filters with deionized water prior to use.
5.4.3. If possible, wipe a surface area covering 100
cm2.
5.4.4. Fold the wipe sample with the
exposed side in.
5.4.5. Transfer the wipe sample into a
20-mL scintillation vial and seal with vinyl or electrical tape.
Securely wrap an OSHA-21 seal
length-wise from vial top to bottom.
5.5. Shipment
5.5.1. Document the operation
sampled and record other chemical substances in use.
5.5.2. Request fluoride analysis. Also request hydrogen
fluoride analysis if the impregnated backup pad was used. For
smear tabs, only total particulate fluoride will be analyzed and
reported.
5.5.3. Submit at least one blank sample with
each set of air or wipe samples. The blank sample should be
handled in the same manner as the other samples except that an
actual sample is not taken.
5.5.4. Ship the samples to
the laboratory for analysis as soon as possible in a suitable
container designed to prevent damage in transit.
6.
Analysis
6.1. Safety Precautions
6.1.1. All work with
concentrated acids or bases is potentially hazardous. Always
wear safety glasses and protective clothing.
6.1.2.
Prepare all fusions in an exhaust hood.
6.1.3. Care
should be exercised when handling any acidic or basic solutions.
If any acid or base contacts the eyes, skin, or clothes, flush
the area immediately with copious amounts of water. Medical
treatment may be necessary. Acid or base contact with work
surfaces should be avoided.
6.1.4. Use a pipet bulb,
never pipet by mouth.
6.1.5. Before using any
instrument, the operator should consult the Standard Operating
Procedure (SOP) (8.5.) and any instrument manuals.
6.2. Equipment
6.2.1. Ion Specific Electrode
(ISE) and filling solution, fluoride (Model 96-09,
Orion Research Inc., Cambridge, MA).
6.2.2. Electrode,
pH and filling solution (Model 81-02 RossTM
Combination pH electrode, Orion Research Inc.).
6.2.3.
Reference electrode and filling solution (Model 90-01, Orion
Research Inc.).
6.2.4. Millivolt/pH meter, capable of
relative mV, pH, standard addition or concentration measurements
(Model EA 940 Expandable Ionanalyzer, Orion Research Inc.).
6.2.5. Stirrer, electronic, or magnetic with Teflon
stirring bars.
6.2.6. Drying oven, vacuum-assisted
(Model 5851, National Appliance Co., Portland, OR).
6.2.7. Nickel or monel crucibles, 75-mL.
6.2.8.
Laboratory glassware including 25-, 250-, 500-, and 1,000-mL
volumetric flasks, and various sizes of Class A volumetric
pipets.
6.2.9. Polyethylene beakers, 100-mL.
6.2.10. Forceps.
6.2.11. Desiccator.
6.2.12. Eyedroppers or disposable Pasteur pipets.
6.2.13. Automatic pipets, adjustable, 0.1- to 5.0-mL
range (models P-1000 and P-5000,
Rainin Instruments Co., Woburn, MA).
6.2.14.Analytical
balance (0.01 mg). 6.3.
Reagents (All chemicals should be reagent grade or
better)
6.3.1. Deionized water (DI
H2O).
6.3.2. Sodium hydroxide (NaOH) pellets.
6.3.3. Sodium hydroxide, 5 N: Dissolve 200 g NaOH
pellets in approximately 600 mL of DI H2O and dilute
to 1-L. Store in a polyethylene bottle.
6.3.4. Sodium hydroxide, dilute: 0.5 and 0.05 N
for pH adjustments.
6.3.5. Sodium fluoride (NaF).
6.3.6. Sodium fluoride stock solution, 1,000 µg/mL as
F¯: Dissolve 2.2105 g NaF in DI H2O and dilute
to 1-L. Store in a polyethylene bottle.
6.3.7. Tris(hydroxymethyl)aminomethane
[(CH2OH)3CNH2].
6.3.8.
Hydrochloric acid (HCl), concentrated (36.5 to 38% w/w).
6.3.9. Hydrochloric acid, dilute (2%) for pH
adjustments.
6.3.10. Sodium tartrate
(Na2C4H4O6·
2H2O).
6.3.11. Tris-Tartrate (T-T) buffer
(concentrated): To approximately 500 mL DI H2O
add 84 mL of concentrated HCl, 242 g
tris(hydroxymethyl)aminomethane and 230 g sodium tartrate. Stir
to dissolve and let cool to room temperature. Dilute to
1-L with DI H2O. Use this concentrated
solution to prepare dilute T-T buffer. Do not use
concentrated T-T buffer in any samples or standards.
6.3.12. Tris-Tartrate (T-T) buffer (analytical): Dilute
360 mL of concentrated T-T buffer to 2-L with DI
H2O. Use this buffer with an equal volume of sample
or standard solution for analysis.
6.3.13. 1:1 T-T
buffer/DI H2O (for dilutions only): Dilute
equal volumes of analytical T-T buffer with DI
H2O. Use this buffer only for
sample dilutions (i.e. when the sample displays an mV reading
above the largest standard).
6.3.14. Buffer solutions,
in the range of pH 4 to 10. 6.4. Standard Preparation
Prepare dilutions
of the 1,000 µg/mL F¯ stock standard. Use DI H2O as the
diluent and store all standards in polyethylene bottles. An
example of preparation of three standards in the analytical
working range is shown:
| Standard |
Dilution of 1,000 µg/mL
Stock Standard |
| 80. µg/mL |
20 mL to 250 mL |
| 40. µg/mL |
20 mL to 500 mL |
| 5.0 µg/mL |
5 mL to 1,000
mL |
6.5. Sample Preparation
6.5.1. Rinse all glassware and
crucibles with 10% HNO3 and DI H2O. Rinse
all plasticware with DI H2O. Allow labware to air dry
before using.
6.5.2. MCE filters
Prepare filters (also wipe smear tabs) suspected of
containing particulate fluoride as follows:
- Carefully remove each filter from it's cassette using a
forceps. Place each filter in a separate, labeled
75-mL nickel or monel crucible. Record the
crucible number in a log book next to the appropriate sample
number. Using an automatic or glass volumetric pipet,
carefully add 5 mL of 5 N NaOH to each crucible.
- Turn on the vacuum-assisted drying oven. Place the heat
setting to maintain a drying temperature of 140 °C (Note:
For the equipment mentioned in Section 6.2.6., 140 °C
is approximately 85% of the maximum setting). Place the
samples on a metal tray and secure with tape to prevent the
crucibles from "walking" off the surface due to vibration.
Place the tray containing the samples on the bottom of the
drying oven. Close the oven door tightly and dry for 1 h at
140 °C.
- Before proceeding, make sure the drying oven air vents are
open to the surrounding atmosphere. Turn on the vacuum to the
drying oven and then close the vents. Continue drying with the
vacuum on for an additional hour. When drying is complete,
open the oven air vents. When the vacuum reaches 10 mmHg or
less, close off the vacuum. Open the oven door when the
internal oven pressure is equal to ambient atmospheric
pressure. Carefully remove the tray of samples and turn off
the oven.
- Place dried samples in a desiccator until the next step
(5) is performed, since the samples will reabsorb moisture
from the air.
- In an exhaust hood, fuse the samples with the added NaOH
by carefully heating the crucible over a Bunsen burner and
slowly swirling the molten NaOH around the inside of the
crucible.
Note: If splattering occurs, place the crucibles back into the
oven for more complete drying.
- Allow the samples to cool, then add 10 mL DI
H2O to each crucible.
- Carefully add 1.5 to 2 mL of concentrated HCl to
neutralize the basic solution.
- Quantitatively transfer the contents of each crucible into
separate 25-mL volumetric flasks and dilute to
volume with DI H2O.
6.5.3. Chemically-treated backup
pads
| Note: |
The MCE filter should always be prepared
and analyzed even if the industrial hygienist specifies
only an HF analysis. See Section 7.3. for more
details. |
Place each impregnated backup pad into a clean
100-mL polyethylene beaker and add 25 mL of DI H2O.
Allow the pads to desorb for at least 1 h. Agitate each solution
occasionally while desorbing. 6.6. Instrument Set-up
Follow the manufacturers' instructions or the SOP (8.5.)
for operation of the analytical instrument and electrodes. Use a
battery-powered or electronic stirrer with stirring
bar to stir any sample or calibration solution (Note: If a
magnetic stir bar is used, make sure the bar does not contact the
electrodes).
6.6.1. Connect the pH electrode (and reference
electrode, if necessary) to the millivolt/pH meter. Calibrate
the instrument using two buffers in the pH 4 to 10 range.
6.6.2. Individually adjust the pH of each sample to
within 4.5 to 10.5 using an eyedropper or Pasteur pipet with
dilute HCl or NaOH as needed.
| Note: |
Do not use a large amount of solution to
adjust the pH. A few drops should be sufficient. The
desorbed solutions from the backup pads normally should
not need any adjustment to achieve a pH within 4.5 to
10.5. |
Rinse the electrode after each standard or sample
measurement.
6.6.3. While adjusting the pH of the
samples, periodically check the instrument for drift by
measuring the pH buffers.
6.6.4. Connect the fluoride
ISE and reference electrode leads to the appropriate sites of
the instrument, place the electrodes in a standard solution, and
allow to stabilize. 6.7. Analytical Procedure
6.7.1. Prepare working standards immediately before
analysis as follows: Pipet 25 mL of the 80-, 40-, and
5-µg/mL standards into 100-mL
polyethylene beakers. Add 25 mL of dilute T-T
buffer to each polyethylene beaker. Depending on the size of the
sample set, prepare enough standards to analyze at the
beginning, during, and at the end of the analysis. A fresh
standard should be analyzed periodically throughout the
analysis.
| Note: |
The total microgram content of the three
standards is 2,000, 1,000, and 125 µg, respectively. Other
standards in this range may be used if desired.
|
6.7.2. Quantitatively transfer samples
from their volumetric flasks into 100-mL
polyethylene beakers. Pipet 25 mL of the dilute T-T buffer into
each empty volumetric flask and then transfer these rinses into
each corresponding beaker.
6.7.3. Using the mV scale on
the millivolt/pH meter, scan each sample and compare the mV
reading to the standards. Always rinse the ISE with DI
H2O and blot dry with a clean dry tissue before
placing it in the next solution to be analyzed. If any mV
reading is lower (less negative) than that of the highest
standard, the sample is above the calibration curve and
therefore must be diluted.
6.7.4. If a sample appears to
be greater than the PEL, the sample may be split into two
aliquots, which can be diluted and analyzed separately. To
decide whether a sample should be split for duplicate analyses,
estimate the concentration of the sample using data presented in
the Appendix. For example, if the sample mV reading is near the
1,000 µg standard and the air volume of the sample is near 200
L, then the estimated air concentration is about 5
mg/m3.
| Note: |
This calculation is only an estimate
- it does not include blank corrections, Time
Weighted Average calculations, etc. and is offered only as
a convenience to allow for duplicate sample analyses.
Final assessment of an overexposure is performed by the
industrial hygienist. |
6.7.5. Sample dilutions
To
estimate the approximate concentration of any samples above the
highest standard, apply the following rule: Doubling the
concentration of the analyte will change the initial mV reading
(Eo) by about 18 mV. Therefore, if the sample has an
initial mV reading 18 mV less than the initial mV reading of the
prepared standard, it is twice as concentrated as the standard.
Similarly, a sample reading 36 mV lower than the standard is
four times as concentrated. For samples that are 18 mV or less
below the highest standard, pipet a 25-mL aliquot
of the sample/T-T buffer mixture into a clean
beaker. Add 25 mL of 1:1 T-T buffer/DI
H2O (Section 6.3.13.). This results in a
two-fold dilution that now is within the analytical
concentration range. If further dilutions are necessary, repeat
the two-fold dilutions until the sample is in the
range of the standards. Save the unanalyzed portion for a
duplicate analysis.
| Note: |
The 1:1 T-T buffer/DI H2O
used for two-fold dilutions is necessary to
maintain a constant ionic strength. |
6.7.6. Place the fluoride and reference
electrodes into a standard solution. Allow the reading to
stabilize and record the reading. Remove the electrodes from the
standard solution, rinse with DI H2O, and blot dry.
Analyze a different concentration standard (usually a ten- to
twenty-fold concentration difference) and determine
the slope from the two readings. Slope values using the
instruments specified in Section 6.2. of this method have been
approximately -56 to -59 mV.
| Note: |
The fluoride ISE can be affected by
changes in temperature. Standards and samples should be at
the same temperature before analyzing. Fluctuations in the
ambient temperature during analysis can sometimes be
compensated for by slope adjustment (see reference 8.4.
for further details). |
6.7.7. If available, use a standard
additions program intrinsic within the instrument to calibrate
and convert readings directly to concentration values. If an
automated program is not available, record the mV reading prior
to standard addition (Eo) and after addition
(Es). The "standard addition" is a 1-mL
aliquot of the 1,000 µg/mL fluoride stock standard (Section
6.3.6.).
6.7.8. Analyze a sample or standard
(Eo). Using a glass or automatic pipet, add a 1,000
µg (as F¯) spike, and then take a final reading (Es
or concentration for automated programs). Follow the SOP for the
particular instrument (8.5.) or manufacturers' guidelines.
Analyze a standard in the concentration range of the samples
after every fifth or sixth sample and at the end of the
analysis. 7. Calculations
7.1. Determine the total g
fluoride content of each sample and blank using a
concentration-response (concentration units versus
µg) linear regression curve if readings were measured in
concentration units.
If mV readings were taken, plot the
mV readings using an appropriate standard additions program. An
example of equations used for standard additions can be found in
reference 8.6. or in ISE manuals.
| Note: |
Recall that 25 mL
of dilute T-T buffer and 1 mL NaF spike is added to each
standard and sample. Since this volume is constant for all
samples and standards, the total µg content of each sample
and standard can be calculated after standard addition
computation as: |
|
|
µg
fluoride = µg/mL fluoride × Solution Volume ×
Dilution Factor |
|
|
Where: |
|
Solution Volume |
= |
Standard or sample volume
(mL) without the addition of T-T buffer and NaF
spike. |
|
Dilution Factor |
= |
Factor from Section
6.7.5. |
7.2. Each air sample is blank corrected and the
concentration is then calculated to determine particulate fluoride
or hydrogen fluoride exposure using the following equations:
Particulate fluoride
| mg/m3 fluoride = |
µg sample - µg
blank
air volume, L |
Where:
µg Sample or Blank = From above
calculation (Section 7.1.)
Hydrogen fluoride
| ppm HF = |
MV × (µg sample
- µg blank)
molecular weight × air volume,
L |
Where:
| MV (Molar Volume) |
= |
24.45 (25 °C and 760 mmHg) |
| µg Sample or Blank |
= |
From Section 7.1. |
| Molecular Weight (HF) |
= |
20.006 |
| Gravimetric Factor |
= |
1.05 |
7.3. Reporting Results
| Note: |
Problems have occurred concerning the
discrimination between particulate and hydrogen fluoride
(See references 8.3. and 8.7. for further details). Past
studies (8.3., 8.8.) have indicated that HF did not
significantly react with the MCE filter, styrene cassette,
or an untreated backup pad before collection on the
chemically-treated backup pad; however, one
study (8.3.) did indicate the possibility of HF reacting
with or being absorbed by particulate on the MCE filter
(especially if the particulate is an adsorbent such as
alumina which is common in aluminum reduction operations). A
recent study (8.7.) appears to indicate some reactivity of
HF with the sampling media components. Due to the
possibility of HF reacting with particulate on the MCE
filter, the potential for underestimating HF exposure
exists. The total fluoride exposure should be considered for
industrial operations having alumina or other adsorbents in
the air during sampling. If possible for TWA determinations,
consecutive samples should be taken over the workshift, not
to exceed 1-h each. This should minimize the
amount of particulate on the MCE filter.
|
Results are reported to the industrial hygienist as
follows:
For particulate fluoride (MCE filters), sample
results are reported as mg/m3 fluoride.
For
chemically-treated backup pad or MFGB samples, results are
reported as ppm hydrogen fluoride. 8.
References
8.1. Hawley, G.G.: The Condensed Chemical Dictionary. 11th ed.
New York: Van Nostrand Reinhold Co., 1987.
8.2. Occupational Safety and Health Administration Technical
Center: OSHA Laboratory Quality Control
Division Data by B. Babcock, Salt Lake City, UT. 1990
(unpublished).
8.3. Einfeld, W., and S.W.
Horstman: Investigation of a dual filter sampling method
for gaseous and particulate fluoride. Amer.
Ind. Hyg. Assoc. J. 40: 626-632 (1979).
8.4. Orion Research Incorporated:
Instruction Manual, Fluoride Electrodes, Model
94-09, Model 96-09. Cambridge, MA: Orion
Research Incorporated, 1977.
8.5. Occupational Safety and Health Administration Technical
Center: Ion Specific Electrode Standard
Operating Procedure. Salt Lake City, UT. In progress
(unpublished).
8.6. Occupational Safety
and Health Administration Analytical Laboratory: OSHA Manual of Analytical Methods edited by
R.G. Adler (Fluoride as F¯ and HF. Method No. VI-3).
Salt Lake City, UT. 1977.
8.7. Lorberau,
C., and K.J. Mulligan: Problem identified with NIOSH method
7902. Appl. Ind. Hyg. 3: 302 (1988).
8.8. Laboratory Services, Worker's
Compensation Board of British Columbia: Hydrogen Fluoride in Air (Analytical Method
No. 0751). Vancouver, B.C., Canada: Worker's Compensation Board of
British Columbia, 1989.
Appendix
Calculated
mg/m3 values for F¯ or
HF |
|
|
|
- - - - - -
- - - - - - - - - - - - - Concn - - - - - - - - - - - - - - -
- - - - - |
|
|
50 |
100 |
250 |
500 |
1,000 |
2,000 |
µg |
|
Air Vol
(L)
|
| 50 |
|
1.0 |
2.0 |
5.0 |
10.0 |
20.0 |
40.0 |
| 100 |
0.5 |
1.0 |
2.0 |
5.0 |
10.0 |
20.0 |
| 150 |
|
0.67 |
1.67 |
3.33 |
6.67 |
13.3 |
| 200 |
0.50 |
1.25 |
2.50 |
5.0 |
10.0 |
| 250 |
|
1.0 |
2.0 |
4.0 |
8.0 |
| 300 |
0.83 |
1.7 |
3.3 |
6.7 |
| 350 |
0.71 |
1.4 |
2.9 |
5.7 |
| 400 |
0.62 |
1.2 |
2.5 |
5.0 |
(mg/m3) |
| 450 |
0.56 |
1.1 |
2.2 |
4.4 |
| 500 |
0.50 |
1.0 |
2.0 |
4.0 |
| 550 |
|
0.90 |
1.8 |
3.6 |
| 600 |
0.83 |
1.7 |
3.3 |
| 700 |
0.77 |
1.4 |
2.9 |
| 800 |
0.71 |
1.2 |
2.5 |
| 900 |
0.67 |
1.1 |
2.2 |
| 1000 |
0.62 |
1.0 |
2.0 |
|
mg/m3 values
are based on the equation:
|
| mg/m3 analyte = |
GF × µg
L air | |
| µg |
= |
estimated reading
obtained (as F¯) |
| GF |
= |
gravimetric factor (1
for F¯, 1.05 for HF) |
| PEL |
= |
2.5 mg/m3 F¯
and 3 ppm HF
|
| Conversion of
mg/m3 HF to ppm values can be accomplished by
multiplying the mg/m3 HF value by
1.222. | |
| |
| |

