|
| Method no.: |
ID-172 |
| |
|
| Matrix: |
Air |
| |
|
OSHA Permissible
Exposure
Limits
Final Rule
Limits:
Transitional
Limit: |
10,000 ppm Time
Weighted Average (TWA)
30,000 ppm Short-Term Exposure Limit
(STEL)
5,000 ppm TWA |
| |
|
| Collection
Procedure: |
Each sample is collected by
drawing a known volume of air into a five-layer gas
sampling bag. |
| |
|
| Recommended Air Volume: |
2 to 5 liters |
| |
|
Recommended Sampling
Rates
TWA
Determinations:
STEL Determinations: |
0.01 to 0.05 L/min
(4 to 8 h sample)
0.3 L/min (15-min sample) |
| |
|
| Analytical
Procedure: |
A portion of the gas sample
is introduced into a gas sampling loop, injected into a gas
chromatograph, and analyzed using a thermal conductivity
detector. |
| |
|
Detection
Limits
Qualitative:
Quantitative: |
200 ppm
500 ppm |
| |
|
Precision and
Accuracy
Validation
Range:
CVT:
Bias:
Overall
Error: |
2,000 to 10,000
ppm
0.026
-0.005
±5.7% |
| |
|
| Special Requirements: |
Samples should be analyzed
within 2 weeks of collection. |
| |
|
| Method Classification: |
Validated Method |
| |
|
| Chemist: |
Rick Cee |
| |
|
| Date (Date Revised): |
1987 (June, 1990) |
| |
|
Commercial manufacturers and products mentioned in this method are
for
descriptive use only and do not constitute endorsements by
USDOL-OSHA.
Similar products from other sources can be
substituted.
|
| |
|
Branch of Inorganic Methods Development
OSHA Technical
Center
Sandy, Utah
|
1.
Introduction
This method
describes the collection and analysis of carbon dioxide
(CO2) in workplace atmospheres. Samples are collected in
gas sampling bags and analyzed using a gas chromatograph (GC).
1.1. History
In the past, the OSHA Salt Lake
Technical Center (OSHA-SLTC) method for analysis of CO2
consisted of a bicarbonate titration using phenolphthalein as the
indicator (8.1.).
The past method suffered from a lack of specificity, possible
contamination from ambient CO2, and a potentially
unsafe collection method. Carbon dioxide was collected in impinger
solutions containing sodium hydroxide.
The most recent
OSHA method for measuring CO2 exposures in the
workplace required the use of detector tubes (8.2.).
Because short-term detector tubes offer only spot checks of the
environment, another method was needed to determine long-term
CO2 concentrations. Other sources advocated the use of
gas chromatography for CO2 analysis (8.3.-8.5.).
This method is similar to the one proposed by NIOSH (8.3.),
with some modifications.
1.2. Principle
For time
weighted average (TWA) or short-term exposure limit (STEL)
determinations, a sampling pump is used to capture a known volume
of air into a five-layer gas sampling bag. A GC fitted with a gas
sampling loop and a thermal conductivity detector (TCD) is then
used to assess sample concentrations of CO2.
1.3. Advantages and Disadvantages
1.3.1. The method is specific for CO2 in
industrial environments. Response characteristics and the
retention time for CO2 lead to positive
identification. Mass spectrometry can be used if additional
verification is necessary.
1.3.2. The method can be used
for ventilation assessments in indoor air quality
investigations. A CO2 level of 1,000 ppm is
considered a determinant of ventilation system performance.
1.3.3. No impinger sampling is required; however gas
sampling bags are used and may be somewhat inconvenient when
handling and shipping.
1.3.4. Changes in humidity do not
affect sample collection.
1.3.5. The bulk of the sample
is not destroyed during analysis; other potentially toxic gases
may also be analyzed from the same sample.
1.3.6. The
gas bags are reusable.
1.3.7. The method requires the
use of a GC with a gas sampling valve.
1.3.8. Analytical
time required per sample is short; elution of CO2,
using stated GC conditions, occurs within 5 min.
1.3.9.
Gas bag samples are only stable for approximately 2 weeks.
Samples should be analyzed as soon as possible. 1.4.
Prevalence and Use
In 1979, CO2 was the 23rd
largest volume chemical produced in the United States (8.6.).
Potential sources for CO2 emission and exposure are
listed:
carbonated beverage manufacturing
carbonic acid
manufacturing
fire extinguisher manufacturing
explosive
manufacturing
municipal water treatment facilities
aerosol
propellant manufacturing
breweries and fermentation
plants
refrigeration units
cloud seeding
greenhouse air
enrichment
lime kilns
by-product of ammonia
production
product of combustion
anode baking
products
fractioning and acidizing of oil
wells
bakeries
grain
elevators
silos
submarines
diving
mining
wells
shielded
arc welding
1.5. Physical and Chemical Information (8.6.,
8.7.):
| Molecular
formula |
CO2 |
| Molecular weight |
44.01 |
| CAS No. |
124-38-9 |
| Appearance |
colorless, odorless gas |
| Taste |
slightly acidic (from reaction
with H2O to for carbonic acid) |
| Flammability |
non-flammable |
| Boiling gravity |
-78.5°C (sublimes) |
| Specific gravity (air = 1) |
1.5240 (0°C) |
| Density |
1.97 g/L (@ STP) |
| Synonymns |
carbonic anhydride
carbonic
acid
gas dry ice |
1.6. Toxicology
Information contained within this section is a synopsis of
present knowledge of the physiological effects of CO2
and is not intended as a basis for OSHA policy.
Carbon dioxide is classified as an asphyxiant gas. In the
atmosphere, CO2 normally exists at concentrations
between 300 and 700 ppm. Larger gas-phase concentrations of
CO2 may produce signs and symptoms of increased
respiratory rate, lassitude, sleepiness, headache, convulsions,
dyspnea, sweating, dizziness, or narcosis. Literature citations
reveal a wide variation in physiological response to exposures at
certain CO2 concentrations (8.6.-
8.10.).
Exposure to CO2 concentrations above 10% are generally
agreed upon as posing an immediate physiologic threat (8.7.-
8.10.).
Inhalation of CO2 can produce physiological
effects on the central nervous, respiratory, and the
cardiovascular systems. Central nervous system (CNS) effects vary
with CO2 concentrations. Signs and symptoms of CNS
involvement include lassitude, drowsiness, narcosis, and
convulsions. At low levels, inhalation of CO2 may cause
a mild depression of the CNS. At approximately 30% CO2
a paradoxical CNS stimulation leading to convulsions and coma is
seen. Carbon dioxide concentrations above 50% induce an anesthetic
effect (8.9.).
Carbon dioxide is a potent stimulator of respiration.
Respiration depth and rate is mainly controlled through
CO2 blood levels. Generalized signs of respiratory
involvement are displayed by shortness of breath, dyspnea,
respiratory acidosis, and a rapid increase in respiratory rate.
Cardiovascular effects of CO2 are demonstrated
by generalized increases in blood pressure, vasodilation, heart
rate, and cardiac output. Peripheral and cerebral vasodilation, as
demonstrated by signs of sweating and headaches, are usually the
first symptoms observed and are prevalent in low concentration
exposures (8.7.,
8.10.).
The signs and symptoms of low level CO2
intoxication are sudden and reversible. Effects of intoxication
are usually apparent within seconds of toxic exposure. After
discontinuation of exposure, signs and symptoms usually dissipate
within a few minutes. 2. Range, Detection Limit, and Sensitivity (8.11.)
2.1. The analytical working range is linear from at
least 200 to 30,000 ppm. The largest standard used during the
study was 30,000 ppm; the response characteristics of the TCD
indicate the upper linear limit may be much larger.
2.2.
The qualitative detection limit was 200 ppm using a 1-mL sample
loop. The quantitative detection-limit is 500 ppm. A lower
detection limit for CO2 can be achieved using a larger
gas-sampling loop; however, ambient CO2 levels are at
least 300 ppm. Evaluation below 500 ppm would most likely be
unnecessary for workplace atmosphere surveillance.
2.3.
The sensitivity of the analytical method (using analytical
conditions stated for a Hewlett-Packard 5730A Gas Chromatograph
and 3385A Automation System) was taken from the slope of the
linear working range curve (200-30,000 ppm range). The sensitivity
is 1.771 area units per 1 ppm. (For the HP 3385A Automation
System, 1 area unit = 3.2 microvolt-second.)
3. Method
Performance (8.11.)
3.1. The pooled coefficient of variation for the
sampling and analytical method was 0.026. The variation was
calculated from data within the range of 2,000 to 10,000 ppm.
3.2. The average recovery of generated samples taken in
the 2,000 to 10,000 ppm range was 99.5%. The range of recoveries
was from 93 to 104%.
3.3. Precision and accuracy data are
derived from generated samples that were aged less than 2 days.
The stability of CO2 in sampling bags is within
precision and accuracy limits up to 14 days after sample
collection.
3.4. Stability tests indicate a significant
loss (>10%) of CO2 when samples are stored longer
than 14 days. Samples should be analyzed as soon as feasible to
minimize storage losses. 4. Interferences
The
gas chromatographic determination of CO2 is relatively
specific; however, any compound having a similar column retention
time and response as CO2 is a potential interference.
4.1. Potential interferences may be minimized by
altering operational conditions such as temperature and column
packings or using gas chromatograph-mass spectrometry as a
secondary source of confirmation.
4.2. Using the
conditions stated within the method, other common gases and vapors
do not present potential interferences. Nitrogen, hydrogen,
oxygen, methane, and carbon monoxide retention times are
significantly less than that of CO2. Chromatograms
showing the elution of various common atmospheric gases and
CO2 are shown in Figures 1
and 2.
5.
Sampling
5.1. Equipment
5.1.1. A personal sampling pump capable of
delivering a flow rate of approximately 0.01 to 0.05 L/min is
necessary for TWA determinations. A larger flow rate pump can be
used for STEL assessments. Either pump must have external inlet
and outlet ports and hose barbs.
5.1.2. Five layer, 5-L
aluminized gas sampling bags are used as the collection media
(the bags can be obtained from OSHA-SLTC or Calibrated
Instruments Inc., Ardsley, NY).
5.1.3. Various lengths
of flexible tubing are used to make pump, sampling media, and
breathing zone connections. 5.2. Sampling Procedure
5.2.1. Calibrate personal sampling pumps. Since the
sampling bags have a total volume capacity of approximately 6 L,
the following sampling scheme for TWA measurements is shown:
Flow
rate (L/min)
|
Hours
sampled
|
Total
sample volume (L)
|
|
| 0.015 |
4 |
3.6 |
| 0.022 |
4 |
5.3 |
| 0.035 |
2.5 |
5.3 |
| 0.050 |
1.5 |
4.5 |
A large flow rate (0.040 to 0.050 L/min) will
require placing new sampling bags into position throughout the
day. For TWA determinations, a flow rate of approximately 0.020
to 0.025 L/min is sufficient for a 4 h sample. For STEL samples,
calibrate the pump to a rate of approximately 0.3 L/min.
5.2.2. Evacuate and check gas sampling bags for leaks.
The sampling bag can be evacuated and leak-tested by applying a
vacuum to the bag. If a vacuum is applied to a leaky sampling
bag, the bag will not fully collapse. If a vacuum pump is not
available, gas sampling bags can be inflated, inspected for
leaks, and then evacuated by hand rolling and flattening.
5.2.3. Label each sampling bag. Attach one end of a
piece of flexible tubing to the inlet hose barb of the pump, and
place the other end in the breathing zone of the worker. Use
another piece of tubing to connect the metal valve sampling bib
of the sampling bag to the outlet hose barb of the pump. A
graphic representation of the pump set-up is shown:
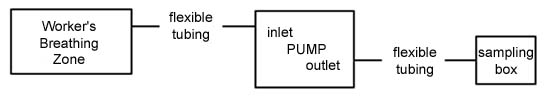 |
| Text Version: The
sampler is composed of an appropriate length of flexible
plastic tubing attached to the inlet of a personal
sampling pump. The other end of this flexible plastic
tubing is placed in the breathing zone of the
worker. Another appropriate length of flexible
plastic tubing is used to connect the outlet of the
sampling pump to the inlet of the sampling
bag. |
5.2.4.
For personal sampling attach the gas sampling bag to any loose
fitting clothing on the worker's back or side using tubing
clamps.
5.2.5. When ready to sample, open the gas
sampling bag valve by rotating the metal valve counter-clockwise
until fully open. Attach the free end of the tubing connected to
the bag to the outlet hose barb. Turn on the pump.
5.2.6. After sampling, rotate gas sampling bag valve
clockwise until tight. Record the total air volume taken.
5.2.7. Do not prepare or submit blank samples. Request
the laboratory analyze for carbon dioxide.
5.2.8. Wrap
an OSHA 21 (or equivalent) seal across the gas sampling bag
valve.
5.2.9. When submitting the sampling bags to the
laboratory for analysis, pack loosely and apply generous padding
to minimize potential damage during shipment. Submit samples as
soon as possible after sampling.
6. Analysis
6.1. Precautions
6.1.1. When preparing CO2 standards, an
Ascarite II filter is used to remove CO2 from the
diluent air. Ascarite II contains sodium hydroxide and silica:
Avoid contact with skin and mucous membranes. Wear gloves and
safety glasses when handling Ascarite II.
6.1.2. The
preparation of CO2 standards should be performed in a
hood. Avoid breathing in any toxic concentrations of
CO2. 6.2. Equipment
6.2.1. A gas chromatograph fitted with a 1-mL
stainless steel gas sampling loop, sampling valves, and a TCD
are used. Loops larger than 1-mL can also be used.
6.2.2. Standard preparation (8.11.):
Due to stability considerations, use only five-layer
aluminum gas sampling bags for standard generation. Gas sampling
bags composed of Tedlar or saran can be used for standards
provided they are prepared and analyzed within 24 h.
6.2.3. Columns:
Chromosorb 102, 6 ft × 1/4 in.
stainless steel, 80/100 mesh. Similar results were obtained
using a 5 ft × 1/4 in. stainless steel, 80/100 mesh, Porapak QS
column.
6.2.4. Data reduction:
An electronic
integrator is used to calculate peak areas.
6.2.5.
Standard generation:
Certified CO2 standards
can be used or standards can be prepared using any combination
of: Calibrated gas-tight syringes or calibrated rotameters, mass
flow controllers, or soap bubble flowmeters. A stopwatch is also
necessary.
6.2.6. Gases:
- If certified standards are not available, undiluted,
bottled CO2 (>99.8%
purity) or pre-diluted CO2 can be used to generate
gas standards. If pure liquid CO2 is used, a
heating tape and variable transformer are necessary for
regulator heating.
- Filtered, compressed, CO2-free air is used for
dilutions. Ambient CO2 is removed from the
compressed air using an Ascarite II/Drierite in-line trap.
(See Precautions in Section 6.1.
before handling Ascarite) Other methods, such as slaked lime
traps, can also be used for removal of ambient CO2.
A diagram of the Ascarite II trap and further information can
be found in reference 8.11.
- Helium (purified) is used as the carrier gas.
6.2.7. Additional accessories:
A personal sampling pump, with an inlet and outlet port
and hose barbs, is used to load the gas sampling loop (loop
loading can also be manually performed by squeezing the sampling
bag). 6.3. Standard
Preparation
6.3.1. Prepare standards by either calibrated
syringe or metered injection of pure or diluted CO2
into the dilution medium. Please see precautions in Section
6.1.2. before preparing.
6.3.2. Completely evacuate and
flush the gas bags used for standard preparation with
CO2-free air (Note: The in-line trap with an Ascarite
II/Drierite bed is used to remove any CO2). Use a
soap bubble flowmeter to measure air flow rates immediately
before and after diluent air addition.
A standard
dilution scheme using pure CO2 is listed for 5-L gas
bags:
ppm
Standard
|
CO2 volume (mL)
|
Airvolume
(mL)
|
|
| Blank |
0 |
4,000 |
| 1,248 |
5 |
4,000 |
| 2,494 |
10 |
4,000 |
| 4,975 |
20 |
4,000 |
| 9,901 |
20 |
2,000 |
| 19,608 |
40 |
2,000 |
Always prepare a blank standard from the
compressed air to account for potential CO2
contamination. Other dilution schemes using smaller or larger
gas bags and gas volumes can be used. Standards should be
prepared in concentrations that bracket the sample
concentrations.
6.3.3. For concentrations other than
those listed above, use the following equation when using pure
or pre-diluted CO2:
| ppm
CO2 = |
(mL
CO2)(1 × 106)(A)
(mL diluent air + mL
CO2) |
where:
| A |
= |
mole fraction or decimal per
cent concentration of the pre-diluted mixture (i.e., for
1.93% CO2 stock standard, A = 0.0193. For pure
CO2, A = 1). |
6.3.4. If a metered generation of standards is
desired, use a mass flow controller or calibrated rotameter to
verify and control the CO2 delivery rate from a gas
cylinder. Use a soap bubble flowmeter immediately before and
after the standard generation to verify the CO2 flow
rate. Meter a known amount of CO2 into a bag already
containing a known volume of CO2-free air. Use a
stopwatch to determine the volume of CO2 delivered
over time.
6.3.5. If using calibrated syringe injection,
fill a gas sampling bag with concentrated CO2 or use
syringe extraction from an in-line gas cylinder septum. Most gas
bags have injection ports or septa for gas syringe withdrawal or
injection. Fill and flush a previously calibrated gas-tight
syringe with pure CO2. Then withdraw and inject the
required volume of CO2 into a gas bag already
containing a measured amount of diluent air.
6.4. Analytical Procedure
6.4.1. Gas chromatograph conditions:
| Helium carrier gas
flow rate |
15 to 25 mL/min |
| Reference gas flow rate |
15 to 25 mL/min |
| Detector, manifold, and column
temperature |
20 to 25°C |
| Retention time |
2 to 6
min |
6.4.2. Sample
and standard introduction:
- Connect the outlet port of the personal sampling pump to
the sampling loop via inert tubing.
- Adjust the pump to give a suitable flow rate for sample
loading from the bag to the sampling loop.
- Connect a short piece of tubing from the inlet port of the
pump to the sample bag. The bag valve is then turned
counterclockwise to the open position and the pump is turned
on.
- After the sample is loaded into the loop, turn the pump
off to allow the loop sample to return to atmospheric
pressure. Wait 1 to 2 min for pressure equalization and then
open the gas sampling valve. Carrier gas flow is now directed
through the sampling loop to the column and detector. (Note:
Samples and standards can be introduced into the loop without
a pump by simply squeezing a sufficient amount of sample from
the bag into the loop. The sampling bag must be released for
loop sample pressure normalization before opening the gas
sampling valve.)
- Perform two determinations of each sample and standard.
6.4.3. If present in the sample,
oxygen, nitrogen, carbon monoxide, and methane will elute before
CO2. Examples of integrated chromatograms of
CO2 and other common gases are shown in Figures 1
and F.
7. Calculations
7.1. If blank correction is necessary for the
standards, subtract blank peak area from standard area readings
before constructing the concentration-response curve. No blank
correction is necessary for the samples.
7.2. Calculate
ppm CO2 concentrations from a linear least-square
regression curve. Establish the regression curve using peak area
(or heights) versus ppm. No calculations using air volumes are
necessary since gas phase samples are compared directly to gas
phase standards. Since the total capacity of the sampling bag is
approximately 6-L, field air volumes can be used by the chemist to
visually assess any leakage during shipment.
7.3. If
necessary, the sample can be analyzed by gas chromatograph-mass
spectroscopy to confirm the presence of CO2.
7.4. Report results to the industrial hygienist as ppm
CO2. 8. References
8.1. Norton, J. F.,
ed.: Standard Methods for the
Examination of Water and Sewage. 9th ed. New York, NY:
America Public Health Association, 1946. pp. 33-40.
8.2. U.S. Dept. of Labor,
Occupational Safety and Health Admin.: Chemical Information File. Online Database --
OSHA Information System. Washington, DC: Directorate of Technical
Support, U.S. Dept. of Labor, OSHA, 1985.
8.3. National Institute for
Occupational Safety and Health: NIOSH
Manual of Analytical Methods. 2nd ed., Vol. 3 (DHEW/NIOSH
Pub. No. 77-157-C). Cincinnati, OH: National Institute for
Occupational Safety and Health, 1977. pp. S249-1-S249-6.
8.4. Katz, M.,
ed.: Methods of Air Sampling and
Analysis. 2nd ed. Washington, D.C.: American Public Health
Association, 1977. pp. 369-373.
8.5.
Guiochon, G. and C. Pommier: Gas Chromatography in Inorganics and
Organometallics. Ann Arbor, MI: Ann Arbor Science Pulishers
Inc., 1973. pp. 80-115.
8.6. Hawley, C.G.: The Condensed
Chemical Dictionary. 10th ed. New York, NY: Van Nostrand
Reinhold Co., 1981.
8.7. National Institute for Occupational Safety and
Health: Criteria for a Recommended
Standard -- Occupational Exposure to Carbon Dioxide
(DHEW/NIOSH Pub. No. 76-194). Cincinnati, OH: National Institute
for Occupational Safety and Health, 1976. pp. 14-114.
8.8. Proctor, N.H. and J.P.
Hughes: Chemical Hazards of the
Workplace. Philadelphia, PA: J.B. Lippincott Co., 1978. pp.
147-148.
8.9. Goodman,
L.S. and A. Gilman, ed.: The
Pharmacological Basis of Therapeutics. 6th ed. New York,
NY: Macmillan, 1980. pp. 331-334.
8.10.
American Conference of Governmental Industrial
Hygienists: Documentation of the
Threshold Limit Values for Substances in Workroom Air. 3rd
ed. Cincinnati, OH: American Conference of Governmental Industrial
Hygienists, 1976. pp. 296-298.
8.11.
Occupational Safety and Health Administration
Technical Center: Carbon Dioxide Backup
Data Report) (ID-172). Salt Lake City, UT. Revised 1990.
| Chromatogram of a Mixture |
Hydrogen
0.9972%
Oxygen 0.9974%
Nitrogen Balance
Carbon Monoxide 1.029%
Methane
0.9972%
Carbon Dioxide
0.9968% | |
|
| RT |
AREA |
AREA
% |
| 0.21 |
1790 |
0.230 |
| 0.63 |
759200 |
97.688 |
| 1.18 |
7566 |
0.973 |
| 2.64 |
8768 |
1.128 | |
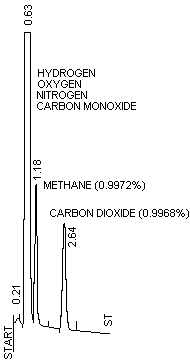 |
| Figure
2 |
|
| |
| |

