|
| Method no.: |
ID-211 |
| |
|
| Matrix: |
Air |
| |
|
OSHA Permissible Exposure
Limits:
Final Rule
Limits:
Transitional
Limit: |
0.3
mg/m3 as sodium azide (Ceiling)
0.1 ppm as hydrazoic
acid (Ceiling)
Also Skin Designation
None |
| |
|
| Collection Device: |
An air sample is collected using a
calibrated sampling pump and a glass tube containing impregnated
silica gel (ISG). A pre-filter is used to collect
particulate azide. Wipe samples can be taken to determine work
surface contamination. |
| |
|
| Recommended Sampling Rate: |
1 liter per minute (L/min) |
| |
|
Recommended Minimum
Sampling
Time: |
5 minutes |
| |
|
| Analytical Procedure: |
The sampling medium is desorbed using
an aqueous solution which contains a mixture of 0.9 mM sodium
carbonate (Na2CO3) and 0.9 mM sodium
bicarbonate (NaHCO3). An aliquot of this solution is
analyzed as azide (N3-) by an ion
chromatograph equipped with a UV detector. |
| |
|
| Special Precautions: |
Ship samples to the laboratory as soon
as possible after collection. Store samples under refrigeration when
not in transit. Samples stored at room temperature need to be
analyzed within 10 days. |
| |
|
Detection
Limit:
Qualitative:
Quantitative: |
0.001 ppm as HN3 or 0.003
mg/m3 as NaN3 (5-L air sample)
0.004 ppm as
HN3 or 0.011 mg/m3 as NaN3 (5-L air
sample) |
| |
|
Precision and
Accuracy:
Validation
Range:
Bias:
Overall
Error:
CVT
(pooled): |
0.057 to 2.63
ppm
-0.022
±12.6%
0.052 |
| |
|
| Method Classification: |
Validated Method |
| |
|
| Chemist: |
James C. Ku |
| |
|
| Date: |
September, 1992 |
| |
|
| Please note: For problems
with accessibility in using figures and illustrations in this
method, please contact the author at (801) 233-4900. |
| |
|
Commercial manufacturers and products mentioned in this method
are
for descriptive use only and do not constitute endorsements
by
USDOL-OSHA.
Similar products from other sources can be
substituted.
|
Branch of Inorganic Methods Development
OSHA Salt Lake
Technical Center
Sandy, Utah
|
1.
Introduction
This method
describes the sample collection and analysis of airborne azides [as
sodium azide (NaN3) and hydrazoic acid (HN3)].
Air samples are taken in the breathing zone of workplace personnel,
and analysis is performed by ion chromatography (IC) with a UV
detector.
Note: Hydrazoic acid vapor may coexist with
NaN3 in the workplace when NaN3 is handled in
the presence of moisture. This method addresses the potential
exposure to both substances (NaN3/HN3), and
may be extended to include other azide compounds, provided they are
soluble in the desorbing solution and collected using the procedure
described below. Wipe or bulk samples can also be collected and
analyzed using this method.
1.1. History
1.1.1. Various sampling and analysis methods have
been proposed in the literature (5.1.
- 5.5.)
for monitoring azide exposures; however, most lack the
sensitivity needed to meet the 0.3 mg/m3 (as
NaN3) or 0.1 ppm (as HN3) Ceiling PEL when
using short sampling periods. Some of these methods are subject
to interferences from many compounds. The ion chromatographic
method has interferences from nitrates or bromides. The National
Institute of Occupational Safety and Health (NIOSH) had proposed
a method for inorganic azide particulates using polyvinyl
chloride (PVC) filter collection followed by water extraction
and IC determination using sodium bicarbonate/sodium hydroxide
eluant (5.6.).
To trap any HN3, NIOSH further recommended using a
solid sorbent tube containing chromosorb coated with sodium
carbonate. The NIOSH method is also subject to interferences and
the conductivity detector used lacks sufficient sensitivity for
short-term samples.
1.1.2. The OSHA Salt Lake Technical
Center (SLTC) previously used a stopgap method for
NaN3 (5.7.).
Samples were collected with impingers which were inconvenient to
use as personal samplers due to possible spillage of the liquid
collection solutions or breakage. Other disadvantages are
similar to those mentioned above: 1) low sensitivity due to the
conductivity detector used; 2) interferences from ions such as
bromide, adipic acid, and nitrate.
1.1.3. It was
desirable to develop a solid-sorbent sampling and analytical
method capable of measuring azide for OSHA compliance purposes.
A method was evaluated using a base-impregnated silica gel (ISG)
as the collection media. The media is similar to that found in
reference 5.5.
1.2. Principle
Particulate NaN3 is
collected on a PVC filter or in the glass wool plug of the
sampling tube. Gaseous HN3 is collected and converted
to NaN3 by the ISG sorbent within the sampling tube.
The collected azide on either media is desorbed in a weak buffer
solution. The resultant anion, N3-, is
analyzed by IC using a variable wavelength UV detector at 210 nm.
A gravimetric conversion is used to calculate the amount of
NaN3 or HN3 collected.
1.3.
Advantages and Disadvantages
1.3.1. This method has adequate sensitivity to
determine compliance with the OSHA Ceiling PEL azide exposures.
1.3.2. The method is simple, rapid, and easily
automated.
1.3.3. The potential for sample contamination
is minimal. The azide anion, N3-, is
normally not detected in sorbent blanks.
1.3.4. One
disadvantage is sample storage stability. Samples should be
refrigerated after collection to improve stability. Samples need
not be refrigerated during shipment provided they are shipped as
soon as possible.
1.3.5. Another disadvantage is the
method does not distinguish azide compounds. If other azide
compounds are present during sampling, and are soluble in the
desorbing solution, positive interferences could occur. However,
most industrial operations do not mix different azide-containing
compounds in their processes. 1.4. Method Performance
A synopsis of the method performance is presented below.
Further information can be found in Section 4.
1.4.1. This method was validated over the
concentration range of 0.057 to 0.263 ppm as HN3. An
air volume of 5 L and a flow rate of approximately 1 L/min were
used.
1.4.2. The qualitative detection limit was 0.00347
µg/mL or 0.0104 µg (as N3-) when using
a 3-mL solution volume. This corresponds to 0.001 ppm
HN3 or 0.004 mg/m3 NaN3 for a
5-L air volume.
1.4.3. The quantitative detection limit
was 0.0116 µg/mL or 0.0348 µg (as N3-) when using
a 3-mL solution volume. This corresponds to 0.004 ppm
HN3 or 0.011 mg/m3 NaN3 for a
5-L air volume. A 50-µL sample loop
and a detector setting of 0.01 absorbance unit (AU) full-scale
output were used.
1.4.4. The sensitivity of the
analytical method, when using the instrumental parameters listed
in Section 3.7.,
was calculated from the slope of a linear working range curve
(0.1 to 1.0 µg/mL
N3-). The sensitivity was 2.1 ×
107 area units per 1 µg/mL.
A Dionex Series 4500i ion chromatograph with AI450 computer
software was used (Dionex, Sunnyvale, CA).
1.4.5. The
precision and accuracy results are shown below (OE = Overall
Error):
| |
Ceiling |
| CV |
0.052 |
| Bias |
-2.2% |
| OE |
±12.6% |
1.4.6. The collection efficiency at 2 times the
PEL was 100%. Samples were collected from a generated test
atmosphere of 0.26 ppm HN3 for 5 min.
1.4.7.
A breakthrough test was performed at a concentration of 0.9 ppm
HN3. Breakthrough was not found when using a sampling
time of 30 min and an average sample flow rate of 1 L/min.
1.4.8. Tests indicated the recovery for samples stored
at room temperature (20 to 25°C) gradually decreases to between
75 and 80% after 30 days. Slight losses (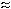 6%) were
observed for samples stored 30 days in a refrigerator or
freezer. 6%) were
observed for samples stored 30 days in a refrigerator or
freezer. 1.5.
Interferences
1.5.1. Other azide compounds will interfere in the
analysis of N3- if they are collected by
the ISG, glass wool, or on the PVC pre-filter.
These compounds should normally not exist in industrial
operations which specifically use NaN3 or
HN3 (i.e. manufacture of air bags, analytical
laboratories, etc.).
1.5.2. Any substance that has the
same retention time and absorbs UV at 210 nm, when using the ion
chromatographic operating conditions described in this method,
may be an interference. If the possibility of an interference
exists, changing the separation conditions (column, eluant flow
rate, eluant concentration, analytical wavelength, etc.) may
circumvent the problem. 1.6. Sources of Exposure
Note: Because NaN3 is rapidly converted to
HN3 on contact with moisture, HN3 is
believed to be the ultimate toxic agent in humans exposed to
NaN3 (5.8.).
Sodium azide has been used for a wide variety of military,
laboratory, medical, and commercial purposes. While it is not
explosive under normal conditions, NaN3 is commonly
used in detonators and other explosives. Sodium azide is used
extensively as an intermediate in the production of lead azide.
The biological uses of azides include inhibition of respiration,
differential selection procedures for bacteria, and bacteriocidal
agents in diagnostic products (5.9.,
5.10.).
Sodium azide is also the chief chemical used to inflate
safety airbags in automobiles. Nitrogen gas is produced upon
NaN3 detonation. After inflation, a small residue of
sodium hydroxide may be left, in addition to lubricants such as
corn starch or talc.
1.7. Physical and Chemical Properties
(5.11.,
5.12.)
Hydrazoic acid (CAS No. 7782-79-8) is a colorless,
volatile liquid which is soluble in water. It has a pungent
obnoxious odor.
| Chemical name |
Hydrozoic
acid |
| Synonym name |
Hydrogen azide |
| Chemical formula |
HN3 |
| Structural formula |
H-N=N N N |
| Formula weight |
43.03 |
| Freezing point |
-80°C |
| Boiling point |
37°C |
Sodium azide (CAS No. 26628-22-8) is a colorless,
hexagonal crystalline solid. It is soluble in water or liquid
ammonia, slightly soluble in alcohol, and insoluble in ether. It
is highly toxic and presents a severe explosion risk when shocked
or heated. When heated to 275 to 330°C in dry air, the solid
crystals decompose with the evolution of nitrogen gas, leaving a
residue of sodium oxide. Sodium hydroxide forms in moist
air.
| Chemical name |
Sodium azide |
| Synonym name |
Sodium azoimide |
| Chemical formula |
NaN3 |
| Structural formula |
Na-N=N N N |
| Formula weight |
65.01 |
| Decomposition temperature |
300°C |
| Specific gravity |
1.846 (@
20°C) |
1.8.
Toxicology (5.13.)
Information listed within this section is a
synopsis of current knowledge of the physiological effects of
NaN3 and is not intended to be used as a basis for OSHA
policy.
Sodium azide/hydrazoic acid is known to
produce hypotension (low blood pressure) in laboratory animals and
humans, and to form strong complexes with hemoglobin, and
consequently block oxygen transport in the blood.
Acute
inhalation of HN3 vapor by humans (which forms when
NaN3 contacts water) results in lowered blood pressure,
eye irritation, bronchitis, headache, weakness, and collapse. A
skin designation has been assigned to the OSHA PEL due to the
ability of NaN3 to readily penetrate intact skin, and
any dermal exposure can significantly contribute to the overall
exposure to azide. 2. Sampling
2.1. Equipment - Air Samples
2.1.1. Calibrated personal sampling pumps capable of
sampling within ±5% of the recommended flow rate of 1 L/min are
used.
2.1.2. Solid sorbent sampling tubes containing ISG
are prepared by using clean silica gel impregnated with a base.
The sampling tube is proprietary and is composed of a glass
jacket containing a 150-mg ISG front and 75-mg ISG backup
section (Cat. No. 226-55, SKC Inc., Eighty Four, PA). The
dimensions of the tube are 7-mm o.d., 5-mm i.d., and 70-mm long.
The ISG is held in place with glass wool and a stainless steel
retainer clip. A pre-filter/cassette sampling
assembly should be used with this tube. See Section 2.1.5.
for more details regarding the pre-filter.
2.1.3. A stopwatch and bubble tube or meter are used to
calibrate pumps.
2.1.4. Various lengths of polyvinyl
chloride tubing are used to connect sampling tubes to pumps.
2.1.5. Anytime the workplace air
being sampled is suspected of containing NaN3, use
the pre-filter/cassette assembly listed below.
a. PVC membrane filter, 37-mm, 5-mm pore
size, [part no. 625413, Mine Safety Appliances (MSA),
Pittsburgh, PA or cat. no. P-503700, Omega Specialty
Instrument Co., Chelmsford, MA]
b. Polystyrene
cassette, 37-mm diameter.
c. Spacer support pad (cat.
no. 225-23, SKC Inc.) (use a spacer in place of a backup pad
to hold the PVC filter securely in the cassette.)
Assemble the pre-filter and sampling
tube such that sampled air enters the cassette first. Use a minimum amount of tubing to connect
the sampling tube to the cassette.
2.1.6. Optional: Desorbing solution (0.9 mM
Na2CO3 + 0.9 mM NaHCO3):
Dissolve 0.191 g Na2CO3 and 0.151
g NaHCO3 in 2.0 L deionized water.
Note: This solution is only used if a delay in sample shipment
is expected.
2.2. Equipment - Wipe Samples
Note: Do not use wipe materials such as smear tabs or those
composed of cellulose; preliminary tests indicate azide is
unstable on this media (recovery was about 50%). Recoveries of
NaN3 spiked on glass fiber or PVC filters were
adequate.
Use either a polyvinyl chloride (PVC) membrane filter, 37-mm,
5-mm pore size, [part no. 625413, Mine Safety Appliances (MSA),
Pittsburgh, PA or cat. no. P-503700, Omega Specialty Instrument
Co., Chelmsford, MA] or a glass fiber filter, 37-mm, (part no.
61715, Gelman Instrument Company, Ann Arbor, MI). Also see the
scintillation vial specification in Section 2.3.
2.3. Equipment - Bulk Samples
Scintillation vials, 20-mL (part no. 74515 or 58515,
Kimble, Div. of Owens-Illinois Inc., Toledo, OH) with
polypropylene or Teflon® cap liners. If possible,
submit bulk or wipe samples in these vials. Tin or other metal cap
liners should not be used because the metal and azide may react.
2.4. Sampling Procedure - Air Samples
Very few industrial operations are conducted where HN3
exists and NaN3 does not. The tube is used to capture
the HN3 while the filter will capture NaN3.
Particulate NaN3 can be captured in the glass wool plug
of the tube; however, a pre-filter is more effective
in capturing the particulate.
2.4.1. Connect the cassette/tube assembly to the
calibrated sampling pump. Ensure that sampled air will enter the
tube following the direction of the arrow sign (--->) stamped
on the outer glass. Place the sampling device on the employee
such that air is sampled from the breathing zone.
2.4.2.
Use a flow rate of 1 L/min and a minimum sampling time of 5 min.
Take additional samples as necessary.
2.4.3. After
sampling, place plastic end caps tightly on both ends of the
tube and the filter cassette. Apply OSHA Form 21 seals. Record
the sampling conditions such as sampling time, air volume, etc.
on the OSHA 91A form. When other compounds are known or
suspected to be present in the air, record such information and
transmit with the samples. See note in Section 2.7.,
regarding sample shipment.
2.4.4. Use the same lot of
ISG tubes and PVC filters for blank and collected samples.
Prepare and handle the blank sorbent tube(s) and filter
cassette(s) in exactly the same manner as the sample tubes
except that no air is drawn through blanks. 2.5.
Sampling Procedure - Wipe Samples for Sodium Azide Particulate
A skin designation has been assigned by OSHA to these
azide-containing compounds.
2.5.1. Wear clean, impervious, disposable glove when
taking each wipe sample.
2.5.2. DO
NOT moisten the wipe PVC or glass fiber filters with
deionized water prior to use. Use a dry
filter to wipe for surface contamination of azide
compounds.
2.5.3. If possible, wipe a surface area
covering 100 cm2.
2.5.4. Fold the wipe filter
sample with exposed side in. See note in Section 2.7.,
regarding sample shipment.
2.5.5.
Transfer the wipe sample into a 20-mL scintillation vial and
seal with vinyl tape. Securely wrap an OSHA-21 seal length-wise
from vial top to bottom.
2.5.6. Prepare a blank wipe
sample by placing an unused wipe filter sample in a
scintillation vial. Seal the vial as discussed in Section 2.5.5.
2.6. Sampling Procedure - Bulk Samples
2.6.1. Take a representative sample of the bulk
material in the workplace. Transfer the bulk material into a
20-mL scintillation vial and seal with vinyl or electrical tape.
Securely wrap an OSHA-21 seal length-wise from vial top to
bottom.
2.6.2. The type of bulk sample should be stated
on the OSHA 91A and cross-referenced to the appropriate air
sample(s). 2.7. Shipment
Note: If a delay in shipment is anticipated (> 2 days after
taking samples), remove the PVC filters from the cassettes and
place in individual scintillation vials. Add 5.0 mL of desorbing
solution (Section 2.1.6.)
to each scintillation vial containing a PVC filter. Add 10 mL of
desorbing solution to each scintillation vial containing a wipe
filter sample. Refrigerate any tube samples until shipment.
2.7.1. Submit at least one blank sample with each
set of air or wipe samples.
2.7.2. Send the samples to
the laboratory as soon as possible
with the OSHA 91A paperwork requesting total azide analysis.
2.7.3. Bulk samples should be shipped separately from
air samples. They should be accompanied by Material Safety Data
Sheets if possible. Check current shipping restrictions and ship
to the laboratory by the appropriate method.
3. Analysis
Note: Upon receipt by the laboratory, all samples are stored under
refrigeration (~4°C) until analysis. This includes wipe, filter,
sorbent, and bulk samples. Samples inadvertently stored at room
temperature need to be analyzed within 10 days.
3.1. Safety Precautions
3.1.1. Refer to appropriate IC instrument manuals
and the Standard Operating Procedure (SOP) for proper instrument
operation (5.14.).
3.1.2. Observe laboratory safety regulations and
practices.
3.1.3. Sodium azide is highly toxic and
presents a severe explosion hazard if shocked or heated. Use
appropriate personal protective equipment such as safety
glasses, goggles, gloves, and lab coat when handling this
chemical. Prepare solutions in an exhaust hood. Store unused
solutions in a refrigerator or dispose of properly.
3.2. Equipment
Chromatographic equipment which allows for analyte contact with
metal surfaces MAY reduce the amount of azide present. It is
recommended to use equipment in which samples have minimal or no
contact with metal surfaces. Analysts should avoid using metal
spatulas when weighing azide compounds, or IC
pre-column or columns contaminated with heavy metals.
3.2.1. Ion chromatograph (Model 4000i or 4500i
Dionex, Sunnyvale, CA) equipped with a variable UV detector.
3.2.2. Automatic sampler (Dionex Model AS-1) and sample
vials (0.5 mL).
3.2.3. Laboratory automation system: Ion
chromatograph interfaced to a data reduction system (AutoIon
450, Dionex).
3.2.4. Separator and guard columns, anion
(Model HPIC-AS9 and AG9, Dionex).
Note: The pH of the eluant must be maintained between 2-11 and
hydroxide ion must not be present in
significant amounts if Dionex AS9 and AG9 columns are used.
Irreversible damage to the columns (guard and separator column)
will result.
3.2.5. Disposable syringes (1 mL).
3.2.6. Plastic or
Teflon®-coated spatulas used for weighing
NaN3.
3.2.7. Miscellaneous volumetric
glassware: Micropipettes, 10-mL volumetric flasks, 25-mL
Erlenmeyer flasks, graduated cylinders, and beakers.
3.2.8. Scintillation vials, glass, 20-mL, with
polypropylene- or Teflon®-lined caps.
3.2.9.
Equipment for eluant degassing (vacuum pump, ultrasonic bath).
3.2.10. Analytical balance (0.01 mg).
3.2.11.
Exhaust hood. 3.3. Reagents - All chemicals should be
at least reagent grade.
3.3.1. Principal reagents:
| CAUTION: |
NaN3 can
be a dangerous chemical, and can cause an explosion when
shocked or heated. It is also a skin irritant and a
hypotensive agent. Avoid skin contact and handle this
chemical and any solutions with care. Do not dry
NaN3 in a drying oven! |
Sodium carbonate
(Na2CO3)
Sodium bicarbonate
(NaHCO3)
Sodium azide
(NaN3)
Hydrochloric acid
(HCl)
Deionized water (DI H2O) - specific
conductance <10 µS.
3.3.2. Eluent and desorbing solution (0.9 mM
Na2CO3 + 0.9 mM NaHCO3):
Dissolve 0.191 g Na2CO3 and 0.151
g NaHCO3 in 2.0 L DI H2O. Sonicate this
solution and degas under vacuum for 15 min. Prepare weekly.
3.3.3. Azide (N3-) stock standard
(1,000 µg/mL):
Prepare the
azide stock standard in an exhaust hood. Carefully weigh 1.5476
g of NaN3 (Aldrich Chemical Company, Inc., Milwaukee,
WI). Dissolve and dilute to 1.0 L with DI H2O.
Prepare monthly.
3.3.4. Azide
(N3-) standard solutions (100, 10, and 1
µg/mL):
Perform serial
dilutions of the 1,000 µg/mL
N3- stock standard using volumetric pipets
and flasks. Dilute to the mark with eluant. Prepare every two
weeks. The larger standards (100 and 10 µg/mL) can be used as working standards, if
necessary.
3.3.5. Dispose of azide or azide solutions
according to the chemical manufacturer, and local or federal
waste disposal guidelines. A method for disposal of aqueous
azide solutions recommended by the Royal Society of Chemistry
(5.15.)
is to dilute the solution greatly with water and then run to
waste.
| CAUTION: |
Do not dispose of untreated
azides or concentrated azide solutions by pouring down
sink drains. |
3.4. Working Standard Preparation
3.4.1. Prepare N3- working
standards in the ranges specified below:
Working Std
(µg/mL)
|
Std
Solution
(µg/mL)
|
Aliquot
(mL)
|
Eluant Added
(mL)
|
|
| 0.05 |
1.0 |
0.5 |
9.5 |
| 0.10 |
1.0 |
1.0 |
9.0 |
| 0.20 |
1.0 |
2.0 |
8.0 |
| 0.50 |
1.0 |
5.0 |
5.0 |
| 0.75 |
1.0 |
7.5 |
2.5 |
| 1.00 |
1.0 |
* |
* |
|
| *
Already prepared in Section 3.3.4. |
3.4.2. To prepare 10 mL of each working standard,
pipette an appropriate aliquot (Aliquot column listed above) of
the 1.0 µg/mL standard solution into a
scintillation vial or Erlenmeyer flask. Add the specified amount
of eluant (Eluant Added column). As an alternative, pipet each
aliquot into a 10-mL volumetric flask and dilute to volume with
eluant. 3.5. Sample
Preparation - Air Samples
| Note: |
Samples desorbed in the field
(Section 2.7.)
are ready for analysis (Section 3.7.). |
3.5.1. Remove filter cassette
and tube samples from the refrigerator and allow them to warm to
room temperature.
3.5.2. Tube
Samples:
Carefully remove the end glass wool
plug. The sorbent should always be removed from the glass tube
via the opposite end of collection (i.e. backup section is
removed first). This will minimize the possibility of
contamination from any collected particulate.
3.5.3. Transfer each section of the ISG and
glass wool plugs and place in separate 25-mL Erlenmeyer flasks
or 20-mL scintillation vials. Place the front glass wool plug
and front ISG section (150 mg) in one container and place the
middle and end glass wool plug in another container with the
backup ISG section (75 mg).
3.5.4. Pipette 3.0 mL of
desorbing solution into each container. Cap each flask tightly
and allow the solution to sit for at least 60 min. Swirl the
solution occasionally.
3.5.5. Filter
Samples:
Carefully remove each filter from the
cassette and place into individual 20-mL scintillation vials.
Add 5.0 mL of desorbing solution to each vial. Cap each vial
tightly and allow the solution to sit for at least 60 min. Swirl
the solution occasionally. 3.6. Sample Preparation - Wipe and Bulk Samples
Note: Samples desorbed in the field (Section 2.7.)
are ready for analysis (Section 3.7.).
3.6.1. Remove wipe and bulk samples from the
refrigerator and allow them to warm to room temperature.
3.6.2. Weigh out representational
aliquots of bulks.
3.6.3. Carefully transfer wipe
samples, and previously weighed aliquots of bulk samples to
separate labeled 20-mL scintillation vials and add 10.0 mL of
desorbing solution into each vial. Cap each vial tightly and
allow the solution to sit for at least 60 min. Swirl the
solution occasionally. 3.7. Sample
Analysis
3.7.1. Pipette a 0.5- to 0.6-mL portion of each
standard or sample solution into separate automatic sampler
vials. Place a filtercap into each vial. The large filter
portion of the cap should face the solution.
3.7.2. Load
the automatic sampler with labeled samples, standards, and
blanks.
3.7.3. Set up the ion chromatograph in
accordance with the SOP (5.14.)
Typical operating conditions for a Dionex 4500i with a
variable wavelength UV detector and an automated sampler are
listed below:
| Ion Chromatograph |
|
| Eluant: |
0.9 mM
Na2CO3/0.9 mM
NaHCO3 |
| Column temperature: |
ambient |
| Anion
pre-column: |
AG9 |
| Anion separator column: |
AS9 |
| Variable UV wave length: |
210 nm |
| Variable UV output range: |
0.01 AU |
| Sample injection loop: |
50 µL |
|
| Pump |
|
| Pump pressure: |
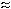 500
psi 500
psi |
| Flow rate: |
1 mL/min |
|
| Chromatogram |
|
| Run time: |
12 min |
| Peak retention time: |
8 to 9 min for
N3- |
3.7.4. Follow the SOP for further instructions
regarding analysis (5.14.).
3.8. Calculations
3.8.1. After the analysis is completed, retrieve the
peak areas or heights for the azide anion. Obtain hard copies of
chromatograms from a printer. An example chromatogram of a solid
sorbent sample collected at an hydrozoic acid concentration of
approximately 2 × PEL is shown below:
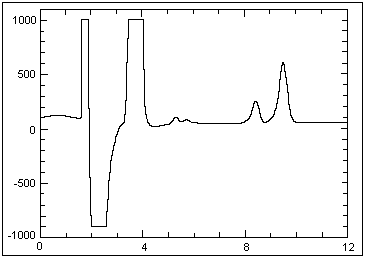
Example chromatogram of a solid sorbent
sample collected at an hydrozoic acid concentration of
approximately 2 × PEL |
|
| Sample Name:
AZS-124 |
Detector:
VDM-1 |
|
| REPORT |
VOLUME |
DILUTION |
POINTS |
RATE |
START |
STOP |
AREA |
REJECT |
|
| External |
1 |
1 |
3605 |
5Hz |
0.00 |
12.02 |
100000 |
|
Peak
No. |
Retention
Time (Min) |
|
Component
Name |
|
|
--------Peak--------- |
|
| 1 |
0.77 |
|
28,132 |
1,527,608 |
| 2 |
1.67 |
|
1,193,355 |
26,810,606 |
| 3 |
3.48 |
|
612,318 |
19,049,090 |
| 4 |
5.28 |
|
53,446 |
791,025 |
| 5 |
5.72 |
|
20,583 |
286,812 |
| 6 |
8.42 |
|
Azide |
|
196,012 |
4,072,104 |
| 7 |
9.50 |
|
Nitrate |
|
550,223 |
12,829,040 |
3.8.2. Prepare a concentration-response curve by
plotting the peak areas or peak heights versus the concentration
of the N3- standards in µg/mL.
3.8.3. Calculate the air
concentration of NaN3 (in mg/m3) for each
filter or sorbent sample:
| WSA
= (µg/mL
N3-)(SV)(GF)SA |
|
|
Where:
| WSA |
= |
Total µg of NaN3 in the
sampleWB = Total µg
of NaN3 in the blank sample |
| µg/mL N3- |
= |
Amount found (from
curve) |
| SV |
= |
Solution volume (mL) from
Section 3.5.3.
|
| (GF)SA,
NaN3/N3- |
= |
Gravimetric factor = 1.5476
|
| AV |
= |
Air volume
(L) |
3.8.4.
Calculate the total concentration of NaN3 (in µg) in each wipe or bulk sample using the
appropriate equation:
| µg NaN3 = WSA -
WB |
(Wipe
Samples) |
|
| NaN3 % (w/w) = |
WSA × 100%
S × F | |
(Bulk
Samples) |
Where:
| S |
= |
Sample wt, mg = Aliquot of
bulk taken in Section 3.6.2. |
| F |
= |
1,000 µg/mg | 3.9. Reporting Results
3.9.1. Add the PVC filter and sorbent results
together for each sample. Report this sum result to the
industrial hygienist as mg/m3 NaN3
(total).
| Note: |
Vapor phase and particulate
results should be combined to determine compliance and to
minimize confusion. Although the vapor phase is a ppm
value, the OSHA regulation stipulates "sodium azide" as
sodium azide or as hydrozoic acid (5.13.).
The total exposure to both phases needs to be considered
for compliance and the results need to be reported as
either total mg/m3 NaN3 or total ppm
HN3 to minimize confusion. If it is necessary
to determine the ppm amount of HN3, see the Appendix. |
3.9.2. Wipe sample concentrations are
reported as total micrograms or milligrams of NaN3.
3.9.3. Bulk sample results are reported as approximate
percent by weight sodium azide. Due to differences in sample
matrices between bulks and analytical standards, bulk results
are approximate. 4. Backup Data
This method has been validated
for a 5-L, 5-min sample taken at a flow rate of 1 L/min. The method
validation was conducted near the OSHA Ceiling PEL. The sampling
media used during the validation consisted of
two-section tubes packed with 150-mg of ISG for the
front and also 150 mg for the backup sections. Tubes were obtained
commercially from SKC (Lot no. 782, Cat. no. 226-55, SKC Inc.,
Eighty Four, PA).
| Note: |
After the validation was
completed, the manufacturer reduced the amount of sorbent in
the backup section to 75 mg, and reduced the length of the
sampling tube from 110 mm to 70 mm. This change produces a
smaller, more convenient sampling train
(pre-filter cassette/sampling tube) and should
not affect results. |
The validation consisted of the following
experiments and discussion:
- An analysis of 24 spiked samples was performed (8 samples each
at 2 ×, 1 ×, and 0.5 × the Ceiling PEL) to evaluate desorption
efficiency (DE).
- A sampling and analysis of 18 samples (6 samples each at 2 ×,
1 ×, and 0.5 × Ceiling PEL) collected from dynamically generated
test atmospheres at 50% RH. to determine bias and overall error.
- A determination of the sampling media collection efficiency at
approximately 0.26 ppm (
 2 × Ceiling PEL). 2 × Ceiling PEL).
- A determination of potential breakthrough.
- An evaluation of storage stability at room (20 to 25°C),
refrigerator (0 to 4°C), and freezer (-10 to -14°C) temperatures
for 64 collected samples.
- A determination of any significant effects on results when
sampling at different humidities.
- A determination of the qualitative and quantitative detection
limits.
- Evaluation of a pre-filter/cassette assembly or
foam for use during sampling.
- Determination of stability of NaN3 on wipe sampling
media.
- Summary.
A generation system was
assembled, as shown in Figure
1, and used for all experiments except detection limit
determinations. All samples were analyzed by IC. All known
concentrations of generated test atmospheres were calculated from
impinger samples which contained 1.0 mM
Na2CO3/1.0 mM NaHCO3 solutions.
These impinger samples were taken side-by-side with any ISG samples.
All results were calculated from concentration-response
curves and statistically examined for outliers. In addition, the
analysis (Section 4.1.)
and sampling and analysis results (Section 4.2.)
were tested for homogeneity of variance. Possible outliers were
determined using the Treatment of Outliers test (5.16.).
Homogeneity of variance was determined using Bartlett's test (5.17.).
Statistical evaluation was conducted according to the Inorganic
Methods Evaluation Protocol (5.18.).
The overall error (OE) (5.18.)
was calculated using the equation:
| OEi% = ±(|biasi| + 2CV i) × 100% (95% confidence
level) |
Where
i is the respective sample pool being
examined.
4.1. Analysis
Twenty-four
samples were prepared by adding known amounts of NaN3
(as N3-) stock solution to the ISG tubes to
determine desorption efficiencies (DEs) for the analytical portion
of the method.
4.1.1. Procedure: Sampling
tubes containing ISG were spiked using a 25-µL syringe (Hamilton
Microliter ®/Gastight® Syringe, Hamilton
Co., Reno, NV). Spikes were 0.5, 1.0, and 2.0 µg
N3-. These levels correspond approximately
to 0.5, 1, and 2 times the Ceiling PEL for a 5-L air sample at a
1-L/min flow rate.
4.2.
Sampling and Analysis
To determine the precision and
accuracy of the method, known concentrations of HN3
were generated, samples were collected and then analyzed. A block
diagram of the generation system used is shown in Figure
1.
4.2.1. Procedure:
Test atmospheres of HN3 were generated using a
syringe pump (Model 355 syringe pump, Sage Instruments,
Cambridge, MA) and a dynamic generation system. To prepare the
atmospheric concentrations, a 1,000 µg/mL azide solution
(prepared from NaN3, EM Science, Cherry Hill, NJ)
was used. For each HN3 atmosphere generated, 100 µL
of concentrated HCl was added to 10 mL of the azide solution
to drive the reaction of NaN3 to HN3.
The HCl/NaN3/H2O solution was
immediately loaded into a 10-mL disposable syringe driven
between 0.13 and 0.52 mL/h through 60 cm of a
Teflon® needle (KF30TF needle, Hamilton Co., Reno,
NV) into a glass mixing chamber. The mixing chamber was
connected to a filtered and humidified airstream.
Dynamic generation system
A Miller-Nelson
Research Inc. flow, temperature, and humidity control system
(Model HCS-301, Monterey, CA) was used to control and
condition the airstream. All generation system fittings and
connections were Teflon®. The HN 3
concentrations were varied by either adjusting the dilution
airstream volume or the speed of the syringe pump delivering
the azide. The dilution airstream was adjusted using the mass
flow controller of the Miller-Nelson system. The
system was set to generate test atmospheres at 50% RH and
25°C.
The total flow rate of the generation system was measured
using a dry test meter.
Side-by-side solid-sorbent and impinger samples were taken
from the sampling manifold using constant-flow pumps. Alpha 1
pumps (E.I. Du Pont de Nemours & Co., Wilmington, DE) and
Gilian® Gil-Air SC pumps (Gilian Instrument Corp.,
W. Caldwell, NJ) were used for impinger and ISG samples,
respectively. For the ISG samples, pump flow rates were
approximately 1 L/min and sampling time was 5 min. For
impinger samples, a 1 L/min sampling rate for 15 min was used.
Generation system concentrations were approximately 0.5, 1,
and 2 times the OSHA Ceiling PEL. 4.2.2. An independent source was used
for NaN3 analytical standard preparations (Aldrich
Chemical Company, Inc., Milwaukee, WI). All samples and
standards were analyzed in accordance with Section 3
of this method.
4.2.3. Results: The results are shown in Table 2) and spiked sample (Table
1) results each passed the Bartlett's test and were pooled
to determine a total CV (CVT) for the sampling and
analytical method. For the experiments, the pooled coefficients
of variation, bias, and OE are as follows:
| CV1
(pooled) = 0.023; |
CV2
(pooled) = 0.051 |
|
| CVT (pooled) =
0.052; |
bias = -0.022; |
OE =
±12.6% | 4.3. Collection Efficiency
Procedure: Six
commercially-prepared sampling tubes were used for collection at a
concentration of approximately 2 times the OSHA Ceiling PEL for 5
min at 1 L/min (50% RH and 25°C). The amounts of HN3
vapor collected in the first section (150 mg of sorbent) and
second section (150 mg) were determined. The collection efficiency
(CE) was calculated by dividing the amount of HN3
collected in the first section by the total amount of
HN3 collected in the first and second sections.
Results: The results in Table
3 show a CE of 100%. No HN3 was found in the second
sorbent section for the CE experiment.
4.4. Breakthrough
(Note: Breakthrough is defined as >5% loss of analyte
through the sampling media at 50% RH)
Procedure: The same procedure as the CE
experiment (Section 4.3.)
was used with two exceptions: The generation concentration was
increased to a level approximately 9 times the Ceiling PEL, and
samples were taken at 1 L/min for 30 min.
The amount of
breakthrough for each sampling tube was calculated by dividing the
amount collected in the second section by the total amount of
HN3 collected in the first and second sections.
Results: No breakthrough of
HN3 into the second section was found. Results are
shown in Table
4.
4.5. Storage Stability
Procedure: Two tests were
conducted to assess storage stability. The first was a preliminary
study of storage at room temperature (20 to 25°C) after
HN3 collection. Twenty-four samples were taken near the
OSHA Ceiling PEL of 0.1 ppm. After collection, all samples were
stored under normal laboratory conditions (20 to 25°C) on a lab
bench and were not protected from light. Six samples were
initially desorbed and analyzed, then six samples were desorbed
and analyzed after various periods of storage (5, 15, and 32
days).
An additional test was conducted by generating 40
samples (4 room-temperature samples at day 15 were discarded due
to analytical problems) for a temperature-dependent storage
stability test, including 4 control samples (used for day 0). The
samples were separated into 3 groups and each group consisted of 4
samples per storage period. A group was stored at either room,
refrigerated, or freezer temperature. The same analytical
procedure as the previous storage test was used. Samples were
analyzed after 0, 7, 15, and 30 days.
Results: As shown in Table
5a and the graph below, the results of the first test show the
mean of samples analyzed after 32 days was only 77% of the value
of day 0. Table
5b and the graph below show the results of the second study at
different temperatures. The recovery is only 77% of the value of
day 0 after a 30-day storage at room temperature. This drastic
change was not noted for samples stored at refrigerated or freezer
temperatures; however, a slight decrease in sample recoveries (93
- 94%) after 30 days was apparent.
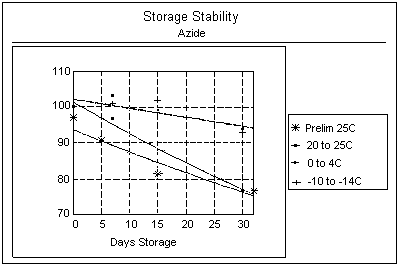
Storage
Stability Results |
4.6. Humidity Study
Procedure: A study was conducted to determine
any effect on results when samples are collected at different
humidities. Samples were taken using the generation system and
procedure described in Section 4.2.
Test atmospheres were generated at 25°C and at approximately 0.5,
1, and 2 times the OSHA Ceiling PEL. Relative humidities of 30%,
50%, and 80% were used at each concentration level tested.
Results: Results of the humidity
tests are listed in Table
6. An F test was used to determine if any significant effect
occurred when sampling at different humidities. As shown, the
calculated F values are less than critical F values (5.19.)
for all the concentrations tested and no significant difference in
results occurred across the humidity ranges tested.
4.7.
Qualitative and Quantitative Detection Limit Study
Procedure: Low concentration samples were
prepared by spiking desorbing solutions (Section 3.3.2.)
with aliquots of aqueous standards prepared from NaN3
(Section 3.3.4.).
These samples were analyzed using a 50-µL sample injection loop and a variable
wavelength UV detector setting of 0.01 absorbance unit (AU). A
derivation of the International Union of Pure and Applied
Chemistry (IUPAC) detection limit equation (5.20.)
was used to calculate detection limits.
4.8. Pre-filter
Evaluation
Procedure: Past
research regarding aerosols (5.21.)
has indicated that particulate in the air sampled may penetrate
any glass wool plugs and the sorbent when using conventional
sampling tubes. A pre-filter can be used to assist in
capturing particulate before entry into the sampling tube. A study
was conducted to evaluate the possibility of HN3
reacting with a pre-filter/cassette or foam sampling
device to capture NaN3 or other particulate.
Evaluations were performed using either ISG sampling tubes with
pre-filter sampling assemblies (PVC filter/spacer
support/cassette), or with polyurethane foam [foam used in the
combination sampling device for SO2 (Type II tube),
OSHA Method No. ID-200] for particulate collection.
A test
was conducted by taking four ISG samples without
pre-filters side-by-side with four ISG samples
connected with pre-filters. This test was repeated
with foam plugs instead of the pre-filters. Samples
were taken such that the test atmosphere entered the
pre-filter or foam first and then entered the ISG.
Short pieces of Tygon® tubing were used to connect the
cassettes and ISG sampling tubes. All samples were taken at a flow
rate of about 0.8 L/min for 5 min. The generation system
concentration was approximately 1.5 times the Ceiling PEL.
Results: The results of the
comparison of ISG samples taken, with and without
pre-filter or foam, are shown in Tables
8a and 8b,
respectively. As shown, a difference in the amount of
HN3 collected was not noted between the
pre-filter/ISG and ISG, or foam/ISG. The PVC
pre-filter/cassette or the foam does not appear to
inhibit the collection of HN3 when using the sampling
conditions stated in Section 2.
The PVC filter/cassette assembly is recommended for particulate
sample collection. The foam may be validated for use in the future
to develop a combination sampling device for collection of both
NaN3 and HN3. The ability of the foam to
effectively capture NaN3 needs to be further examined.
4.9. Stability of NaN3 on Wipe Sampling Media
Procedure: A determination of
the stability of NaN3 was conducted using 37-mm glass
fiber filters (Cat. no. 61715, Gelman Instrument Company, Ann
Arbor, MI) and smear tabs (Lot. no. 3034, Whatman LabSales Inc.,
Hillsboro, OR). The stability of sodium azide on PVC membranes has
been previously reported as stable up to 10 days of storage (5.6.).
Glass fiber filters or smear tabs were spiked using a 25-µL syringe (Hamilton
Microliter®/Gastight® Syringe, Hamilton Co.,
Reno, NV). Solution spikes contained between 7 and 15 µg NaN3. Filters were allowed to
dry and were stored for 3 days on a lab bench, then refrigerated
until analysis.
Results: The
precision and accuracy results for glass fiber filters and smear
tabs are shown below (F/T = Found/Theoretical recovery):
Collection Media
|
|
N
|
|
Mean
(F/T)
|
|
Std
Dev
|
|
CV
|
| Glass Fiber
Filters |
|
5 |
|
1.001 |
|
0.036 |
|
0.036 |
| Smear Tabs |
|
5 |
|
0.412 |
|
0.134 |
|
0.325 |
The recovery data shows that azide is unstable on
cellulose media and stable on glass fiber filters.
4.10.
Summary
The validation results indicate the method meets
both the NIOSH and OSHA criteria for accuracy and precision (5.17.,
5.18.).
Performance during collection efficiency, breakthrough, and
humidity tests is adequate. Although it appears that the recovery
dramatically decreases when storing collected samples at room
temperature after 15 days, no losses were found when storing the
sampling tubes after sample collection in a refrigerator or
freezer. It is recommended to analyze samples within 10 days if
samples are stored without refrigeration and within 30 days if
refrigeration is used. Detection limits are adequate when samples
are taken for 5 min at 1 L/min. The method is adequate for
monitoring occupational exposures to the OSHA Ceiling PEL.
5. References
5.1. Westwood, L.C. and E.L. Stockes: Determination
of Azide in Environmental Samples by Ion Chromatography. Ion Chromatographic Analysis of Environmental
Pollutants, Vol. 2, edited by J.J. Mulick and E. Sawicki.
Ann Arbor, MI. Ann Arbor Science, 1979. p.141.
5.2. Roberson, C.E. and C.M.
Austin: Colorimetric Estimation of Milligram Quantities of
Inorganic Azides. Anal. Chem. 29:854-855
(1957).
5.3. Williams,
K.E., G.G. Esposito, and D.S. Rinehart: Sampling Tubes for
the Collection of Selected Acid Vapors in Air. Am. Ind. Hyg. Assoc. J. 42:476-478 (1981).
5.4. Zehner, J.M. and
R.A. Simonaitis: Gas Chromatographic Determination of
Hydrazoic Acid. J. Chromatogr. Sci.
14:493-494 (1976).
5.5. Puskar, M.A., S.M. Fergon and L.H. Hecker: A
Short-Term Solid Sorbent Determination of Hydrazoic Acid in Air.
Am. Ind. Hyg. Assoc. J. 52(1):14-19
(1991).
5.6. National
Institute for Occupational Safety and Health: Azide particulates (Method No. P&CAM 369).
Cincinnati, OH: National Institute for Occupational Safety and
Health, 1982.
5.7. Occupational Safety and Health Administration Salt Lake
Technical Center: Azide by Ion
Chromatography (Stopgap Method - Unpublished) by J. Germ,
Salt Lake City, UT, 1985.
5.8. National Research Council, Committee on Hazardous
Substances in the Laboratory: Prudent
Practice for Handling Hazardous Chemical in Laboratories,
Washington D.C.: National Academy Press, 1981. pp.145-147.
5.9. Kleinhofs, A.,
W.M. Owais, and R.A. Nilan: Azide. Mutat. Res. 55:165-195 (1978).
5.10. Owais, W.M., A. Kleinhofs,
and R.A. Nilan: In Vivo Conversion of Sodium Azide to a
Stable Mutagenic Metabolite in Salmonella Typhimuriun. Mutat. Res. 68:15-22 (1979).
5.11. Hawley, G.G., ed.
The Condensed Chemical Dictionary, 8th
rev. ed. New York: Van Nostrand Reinhold Co., 1971.
5.12. Yost, D.M., and H. Russell,
Jr.: Systematic Inorganic
Chemistry, New York: Prentice-Hall, Inc., 1946. Ch. 3,
pp.122-131.
5.13. "Sodium azide" Federal Register 54:12 (19 Jan. 1989). pp
2540.
5.14. Occupational Safety and Health Administration Salt Lake
Technical Center: Ion Chromatography Standard Operating
Procedure (Ion Chromatographic Committee). Salt Lake City, UT. In
progress.
5.15. Bretherick, L, ed.: Hazards
in the Chemical Laboratory, 4th ed. London: Royal Society
of Chemistry, 1986. pp. 491-492.
5.16.
Mandel, J.: Accuracy and Precision,
Evaluation and Interpretation of Analytical Results, The Treatment
of Outliers. In Treatise On Analytical
Chemistry. 2nd ed., Vol. 1, edited by I. M. Kolthoff and P.
J. Elving. New York: John Wiley and Sons, 1978. pp. 282-285.
5.17. National
Institute for Occupational Safety and Health: Documentation of the NIOSH Validation Tests by
D. Taylor, R. Kupel, and J. Bryant (DHEW/NIOSH Pub. No. 77-185).
Cincinnati, OH: National Institute for Occupational Safety and
Health, 1977. pp. 1-12.
5.18. Occupational Safety and Health Administration Salt Lake
Technical Center: Evaluation Guidelines
of the Inorganic Methods Branch. In OSHA Analytical Methods
Manual. 2nd ed. Cincinnati, OH: American Conference of
Governmental Industrial Hygienists, 1991.
5.19. Dowdy, S. and S.
Wearden: Statistics for Research.
New York: John Wiley and Sons, 1983. Chapter 8.
5.20. Long, G.L. and J.D.
Winefordner: Limit of Detection – A Closer Look at the
IUPAC Definition. Anal. Chem.
55:712A-724A (1983).
5.21. Fairchild, C.I., and M.I. Tillery: The
Filtration Efficiency of Organic Vapor Sampling Tubes against
Particulates. Am. Ind. Hyg. Assoc. J.
38:277-283 (1977).
Table 1
Azide (as
N3-) Analysis - Desorption Efficiency
(DE)
|
| (OSHA-PEL) |
Taken
(µg N3-) |
Found
(µg N3-) |
DE
(F/T) |
N |
Mean |
Std Dev |
CV |
|
| (0.5 ×
PEL) |
0.500
0.500
0.500
0.500
0.500
0.500
0.500
0.500
|
0.500
0.515
0.520
0.500
0.505
0.510
0.465
0.525 |
1.000
1.030
1.040
1.000
1.010
1.020
0.930
1.050 |
|
|
8 |
1.010 |
0.037 |
0.037 |
| (1 ×
PEL) |
1.000
1.000
1.000
1.000
1.000
1.000
1.000
1.000 |
0.995
0.985
0.995
1.000
1.015
0.995
0.995
1.000 |
0.995
0.985
0.995
1.000
1.015
0.995
0.995
1.000 |
|
|
8 |
0.998 |
0.008 |
0.008 |
| (2 ×
PEL) |
2.000
2.000
2.000
2.000
2.000
2.000
2.000
2.000 |
1.980
1.960
2.000
1.990
1.975
2.010
2.035
1.970 |
0.990
0.980
1.000
0.995
0.988
1.005
1.018
0.985 |
|
|
8 |
0.995 |
0.012 |
0.012 |
|
| F/T = Found/Taken |
|
DE = Desorption
Efficiency |
| CV1 (Pooled) =
0.023 |
|
Average DE =
1.001 | |
| The average DE is very
close to 1.0; therefore, a DE correction is not
necessary. |
Table 2
Hydrazoic Acid Sampling and
Analysis - Ceiling PEL Determination *
(25°C
and 50% RH)
|
| (OSHA-PEL) |
Taken
(ppm
HN3) |
Found
(ppm
HN3) |
Recovery
(F/T) |
N |
Mean |
Std Dev |
CV |
OE
(±%) |
|
| (0.5 ×
PEL) |
0.057
0.057
0.057
0.057
0.057
0.057 |
0.053
0.058
0.053
0.061
0.056
0.054 |
0.930
1.018
0.930
1.070
0.982
0.947 |
|
|
6 |
0.980 |
0.056 |
0.057 |
13.4 |
| (1 ×
PEL) |
1.130
1.130
1.130
1.130
1.130
1.130 |
0.129
0.121
0.135
0.117
0.122
0.121 |
0.992
0.931
1.038
0.900
0.938
0.931 |
|
|
6 |
0.955 |
0.050 |
0.053 |
15.1 |
| (2 ×
PEL) |
0.263
0.263
0.263
0.263
0.263
0.263 |
0.259
0.267
0.252
0.251
0.276
0.272 |
0.985
1.015
0.958
0.954
1.049
1.034 |
|
|
6 |
0.999 |
0.040 |
0.040 |
8.01 |
|
| F/T |
= |
Found/Taken |
| Bias |
= |
-0.022 |
| CV2 (Pooled) |
= |
0.051 |
| CVT (Pooled) |
= |
0.052 |
| Overall Error (Total) |
= |
±12.6% | |
| *Samples were taken for 5
min. |
Table 3
Collection Efficiency
(2 × PEL,
25°C & 50% RH)
|
|
------------------ppm HN3
------------------- |
|
| Sample No. |
First Section |
Second Section |
% Collection
Efficiency |
|
1
2
3
4
5
6 |
0.259
0.267
0.252
0.251
0.273
0.273 |
ND
ND
ND
ND
ND
ND |
100.0
100.0
100.0
100.0
100.0
100.0 |
| Notes: |
- Sampled at 1 L/min for 5 min.
- Samples desorbed using a sample solution volume of
3.0 mL
- ND = None detectable (<0.001 ppm
HN3)
| |
|
Table 4
Breakthrough Study
(25°C and
50% RH)
|
|
------------------ppm HN3
------------------- |
|
| Sample No. |
First Section |
Second Section |
% Breakthrough |
|
1
2
3
4
5
6
7
8 |
0.933
0.940
0.897
0.889
0.938
0.891
0.913
0.925 |
ND
ND
ND
ND
ND
ND
ND
ND |
0
0
0
0
0
0
0 |
| Notes: |
- Sampled at 1 L/min for 30 min
- Due to the large concentration generated and the
analytical sensitivity, the front ISE sections of
sampling tubes were desorbed using larger sample
solution volumes of 10.0 mL.
- ND = None detectable (<0.001 ppm
HN3)
| |
|
Table 5a
Preliminary Test
Storage
Stability - HN3
(1 × PEL, 25°C, and 50% RH)
|
| Day |
Air Vol |
Found |
Taken |
--------------Statistical
Analysis------------- |
|
(L) |
(--ppm
HN3--) |
N |
Mean |
Std Dev |
CV |
Recovery (%) |
|
| 0 |
4.21
4.21
4.21
4.21
4.21
4.21 |
0.129
0.121
0.135
0.117
0.122
0.121 |
0.130
0.130
0.130
0.130
0.130
0.130 |
|
|
6 |
0.124 |
0.007 |
0.053 |
95.4 |
| 5 |
4.21
4.21
4.21
4.21
4.21
4.21 |
0.116
0.124
0.116
0.121
0.117
0.114 |
0.130
0.130
0.130
0.130
0.130
0.130 |
|
|
6 |
0.118 |
0.004 |
0.032 |
90.8 |
| 15 |
3.92
3.92
3.92
3.92
3.92
3.92 |
0.106
0.106
0.107
0.113
0.101
0.101 |
0.130
0.130
0.130
0.130
0.130
0.130 |
|
|
6 |
0.106 |
0.004 |
0.042 |
81.3 |
| 32 |
4.21
4.21
4.21
4.21
4.21
4.21 |
0.096
0.099
0.104
0.100
0.108
0.092 |
0.130
0.130
0.130
0.130
0.130
0.130 |
|
|
6 |
0.100 |
0.006 |
0.057 |
76.8 |
|
Table 5b
Storage Stability -
HN3
at
Room, Refrigerated and Freezer
Temperatures
(Known HN3 Concentration = 0.108
ppm at 50% RH)
|
| Temperature: |
Room
|
Refrigerated
|
Freezer
|
| Day |
Air Vol
(L) |
|
Found
(ppm) |
Air Vol
(L) |
Found
(ppm) |
Air Vol
(L) |
Found
(ppm) |
|
| 0 |
3.05
3.05
3.05
3.05 |
|
0.116
0.120
0.097
0.099 |
*
*
*
* |
*
*
*
* |
*
*
*
* |
*
*
*
* |
|
N
Mean
Std
Dev
CV2
Recovery |
=
=
=
=
= |
4
0.108
0.012
0.108
100% |
|
*
*
*
* |
|
*
*
*
* |
| 7 |
3.54
3.54
3.54
3.54 |
|
0.121
0.115
0.095
0.087 |
3.05
3.05
3.05
3.05 |
0.098
0.126
0.105
0.116 |
3.54
3.54
3.54
3.54 |
0.127
0.108
0.098
0.104 |
|
N
Mean
Std
Dev
CV2
Recovery |
=
=
=
=
= |
4
0.105
0.016
0.154
96.8% |
|
4
0.111
0.012
0.111
103% |
|
4
0.109
0.013
0.115
101% |
| 15 |
+
+
+
+ |
|
+
+
+
+ |
3.54
3.54
3.54
3.54 |
0.120
0.102
0.100
0.106 |
3.05
3.05
3.05
3.05 |
0.129
0.101
0.102
0.110 |
|
N
Mean
Std
Dev
CV2
Recovery |
=
=
=
=
= |
+
+
+
+
+ |
|
4
0.107
0.009
0.084
99.1% |
|
4
0.111
0.013
0.117
102% |
| 30 |
3.54
3.54
3.54
3.54 |
|
0.089
0.076
0.085
0.082 |
3.05
3.05
3.05
3.05 |
0.104
0.103
0.102
0.097 |
3.54
3.54
3.54
3.54 |
0.104
0.091
0.104
0.103 |
|
N
Mean
Std
Dev
CV2
Recovery |
=
=
=
=
= |
4
0.083
0.0055
0.066
76.9% |
|
4
0.102
0.003
0.031
94.0% |
|
4
0.101
0.006
0.063
93.1% |
* Same as
day 0 for room temperature
+ Due to poor precision and
analytical difficulties, data are deleted from statistical
analysis and are not presented graphically in Section 4.5. |
Table 6
Humidity Test -
HN3
(0.5 × PEL & 25°C)
|
% RH
|
30
|
50
|
80
|
| ppm HN3
Taken |
0.061 |
0.057 |
0.057 |
| ppm HN3
Found |
0.068
0.058
0.063
0.057
0.062
0.058
0.062
0.059 |
0.053
0.058
0.053
0.061
0.056
0.054 |
0.057
0.065
0.047
0.067
0.046
0.051 |
N
Mean (ppm)
Std
Dev (ppm)
CV
Ave Recovery |
8
0.061
0.004
0.060
100% |
6
0.056
0.003
0.057
98.0% |
6
0.056
0.009
0.163
97.4% |
|
| At the 99%
confidence level: |
|
|
Fcrit = 6.11 |
Fcalc = 0.12 (2, 17 degrees of
freedom) |
|
| Fcrit > Fcalc; therefore,
no significant difference in results was noted across the
humidity levels tested. |
Humidity
Test - HN3
(1 × PEL & 25°C)
|
% RH
|
30
|
50
|
80
|
| ppm HN3
Taken |
0.124 |
0.130 |
0.121 |
| ppm HN3
Found |
0.122
0.129
0.131
0.125 |
0.129
0.121
0.135
0.117
0.122
0.121 |
0.119
0.124
0.118
0.115
0.115
0.122 |
N
Mean (ppm)
Std
Dev (ppm)
CV
Ave Recovery |
4
0.127
0.004
0.032
102% |
6
0.124
0.007
0.053
95.5% |
6
0.119
0.004
0.031
98.2% |
|
| At the 99%
confidence level: |
|
|
Fcrit = 6.70 |
Fcalc = 3.42 (2, 13 degrees of
freedom) |
|
| Fcrit > Fcalc; therefore,
no significant difference in results was noted across the
humidity levels tested. |
Humidity
Test - HN3
(2 × PEL & 25°C)
|
% RH
|
30
|
50
|
80
|
| ppm HN3
Taken |
0.202 |
0.263 |
0.206 |
| ppm HN3
Found |
0.203
0.214
0.202
0.210
0.200
0.203
0.215 |
0.259
0.267
0.252
0.251
0.276
0.272 |
0.218
0.199
0.213
0.191
0.182
0.196 |
N
Mean (ppm)
Std
Dev (ppm)
CV
Ave Recovery |
7
0.207
0.006
0.030
102% |
6
0.263
0.010
0.040
99.9% |
6
0.200
0.014
0.068
97.0% |
|
| At the 99%
confidence level: |
|
|
Fcrit = 6.23 |
Fcalc = 2.11 (2, 16 degrees of
freedom) |
|
| Fcrit > Fcalc; therefore,
no significant difference in results was noted across the
humidity levels tested. |
Table 7
Qualitative and Quantitative
Detection Limits (IUPAC Method)
|
|
---------HN3 (as
N3-) Level----------- |
|
Sample
No.
|
0.02 µg/mL
PA
|
0.05 µg/mL
PA
|
0.10 µg/mL
PA
|
|
1
2
3
4
5
6
7
|
5.07
5.31
4.65
4.78
4.16
4.42
5.61 |
15.19
17.03
14.10
14.87
12.35
14.41
13.44 |
42.23
36.72
40.02
36.29
39.73
40.21
38.18 |
|
N
Mean
Std
Dev
CV |
7
4.86
0.507
0.104 |
7
14.48
1.468
0.101 |
7
39.05
2.108
0.054 |
PA =
Integrated Peak Area
(N3-)/100,000
The blank and 0.01
mg/mL integrated peak areas, and their standard deviations
(Std Dev) were all equal to zero. |
|
| Using
the
equation: Cld
= k(sd)/m |
| Where:
|
| Cld |
= |
the smallest
reliable detectable concentration an analytical
instrument can determine at a given confidence
level. |
| k |
= |
3 (Qualitative
Detection Limit, 99.86% Confidence) |
|
= |
10 (Quantitative
Detection Limit, 99.99% Confidence) |
| sd |
= |
standard
deviation of the reagent blank (Rbl) readings. |
| m |
= |
analytical
sensitivity or slope as calculated by linear
regression. |
| Cld |
= |
3(0.507)/438.6 =
0.00347 µg/mL as
N3- for the qualitative
limit. |
| Cld |
= |
10(0.507)/438.6
= 0.01156 µg/mL as
N3- for the quantitative limit.
|
Qualitative detection limit = 0.0104 µg N3- (3-mL
sample volume) or 0.001 ppm HN3 (5-L air
volume).
Quantitative detection limit = 0.0348
µg N3-
(3-mL sample volume) or 0.004 ppm HN3 (5-L
air
volume). | |
Table 8a
Comparison Study - With/Without
Pre-filter
(Known Concentration = 0.131 ppm
HN3)
(25°C, and 50% RH)
|
|
With
Pre-filter
|
Without
Pre-filter
|
Sample Set #
|
Air
Vol, L
|
ppm
HN3 Found
|
Air
Vol, L
|
ppm
HN3 Found
|
1
2
3
4 |
3.41
3.41
3.41
3.41 |
0.124
0.140
0.134
0.098* |
3.38
3.38
3.38
3.38 |
0.141
0.138
0.131
0.114 |
N
Mean
Std
Dev
CV
Recovery |
|
3
0.133
0.008
0.061
101% |
|
4
0.131
0.012
0.092
100% |
| * Outlier |
|
| Notes: |
- A 37-mm PVC filter was used as the
pre-filter in a polystyrene cassette.
- Sampling Time = 5 min
- Flow Rate = 0.825 to 0.829 L/min
- Sample Solution Volume for Desorption = 3.0
mL
| |
|
| At the 99%
confidence level: |
|
| tcrit = 9.92
tcalc = -0.615 (2 degrees of freedom) |
|
| tcrit
> tcalc; therefore, no significant
difference in results was noted across the two sets
tested. | |
Table 8b
Comparison Study - With/Without
Foam
(Known Concentration = 0.141 ppm
HN3)
(25°C, and 50% RH)
|
|
With
Foam
|
Without
Foam
|
Sample Set #
|
Air
Vol, L
|
ppm
HN3 Found
|
Air
Vol, L
|
ppm
HN3 Found
|
1
2
3
4 |
3.35
3.35
3.35
3.35 |
0.129
0.140
0.138
0.155 |
3.43
3.43
3.43
3.43 |
0.155
0.135
0.140
0.154 |
N
Mean
Std
Dev
CV
Recovery |
|
4
0.141
0.011
0.077
100% |
|
4
0.146
0.010
0.069
104% |
| Notes: |
- Type II containing 150 mg-ISG glass jacket was
used. The dimensions of the front portion of the glass
jacket are 12-mm o.d., 10-mm i.d., and 25-mm long and
is used for collecting azide particulate. The second
part of the glass tube contains ISG and is used for
collecting HN3. The dimensions of the
second part are 6-mm o.d., 4-mm i.d., and 50-mm long.
Both ends of the sampling tube are sealed with plastic
caps (see Method No. ID-200 for a graphic description
of the Type II glass jacket used).
- Foam analyzed after sampling contained 0.004 ppm
as HN3 (average).
- Sampling Time = 5 min
- Flow Rate = 0.627 to 0.787 L/min
- Sample Solution Volume for Desorption = 3.0
mL
| |
|
| At the 99%
confidence level: |
|
| tcrit = 5.84
tcalc = -0.0055 (3 degrees of freedom) |
|
| tcrit
> tcalc; therefore, no significant
difference in results was noted across the two sets
tested. | |
The system
shown above was used to generate dynamic test atmospheres. The
system consists of four essential elements:
- A flow-temperature-humidity control system,
- An HN3 vapor generating system,
- A mixing chamber, and
- An active sampling manifold.
Appendix
WHA = (µg/mL
N3-)(SV)(GF)HA
| ppm
HN3 = |
WHA × MV
AV ×
MW | |
Where:
| AV |
= |
Air volume |
| WHA |
= |
µg
HN3 |
| (GF)HA,
HN3/N3- |
= |
Gravimetric factor = 1.0238 |
| MV |
= |
Molar volume (L/mol) = 24.45
(25°C and 760 mmHg) |
| MW |
= |
Molecular weight for
HN3 = 43.0 (g/mol) |
| SV |
= |
Solution volume |
| µg/mL
N3- |
= |
Sample result taken from
concentration-response curve | |
| |
| |

